Abstract
Background
Immune checkpoint inhibitors (ICIs) have become a central part of cancer care. However, the survivorship outcomes in patients treated with ICIs are understudied. Therefore, we conducted a scoping review to evaluate the current status of the field and to establish research gaps regarding survivorship outcomes with ICIs in real-life cohorts.
Methods
We used the Web of Science, PubMed, and Embase databases to systematically filter published studies with real-life cohorts from January 1, 2010, until October 19, 2022. Studies evaluating at least one survivorship outcome in ICI-treated patients were included.
Results
A total of 39 papers were included. Quality of life (QoL) (n = 23), toxicity burden (n = 16), and psychosocial issues (n = 9) were the most frequently evaluated survivorship outcomes. Anti-PD-1/PD-L1 monotherapy and a response to treatment were associated with better QoL. In addition, the ICIs were associated with grade 3 or higher immune-related adverse events (irAEs) in 10–15% and late/long-term irAEs in 20–30% of the survivors. Regarding psychosocial problems, over 30% of survivors showed evidence of anxiety and depression, and 30–40% of survivors reported neurocognitive impairments.
Conclusion
The survivors treated with ICIs have impairments in most survivorship domains. Further research is needed to gather data on the understudied survivorship outcomes like late and long-term effects, fertility, financial toxicity, and return to work in survivors treated with ICIs.
Implications for Cancer Survivors
Available evidence demonstrates that a significant portion of survivors treated with ICIs have a significant toxicity burden, lower QoL than the general population, and a high rate of psychosocial problems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) became one of the fundamental anti-cancer treatments [1] in the last decade due to their unique mechanism of action and improved survival outcomes across several tumors. With ipilimumab being the first ICI to obtain regulatory approval in 2011 for the clinical treatment of metastatic melanoma [2], ICIs have entered treatment algorithms in most advanced tumors. These include renal cell carcinoma [3], non-small cell lung carcinoma [4], gastric and esophageal cancers [5], Hodgkin’s lymphoma [6], small cell lung cancer [7], and urothelial cancer [8].
ICIs have a novel mechanism of releasing the immune system’s brakes that could create long-standing disease control and even cure in a significant portion of patients with advanced cancers, especially in tumors with an immune-active milieu like melanoma and non-small cell lung cancer [9, 10]. Recent studies estimated that almost half of the patients with advanced melanoma treated with ICIs in the first line could be cured of the disease [11]. Phase III clinical trials in non-small cell lung cancer [12] and RCC [13, 14] demonstrated over 30% 5-year survival rates with ICIs. Additionally, ICIs have entered the curative adjuvant or neoadjuvant settings for several tumors [15,16,17], further expanding the target population of ICIs. These rapid developments, coupled with the long-standing benefits of ICIs in prolonging survival, have created a new group of survivors of advanced cancer treated with ICIs. While improving survival was the predominant treatment goal in advanced cancer, prolonged survival in the last decade highlighted a need for prioritizing survivorship issues like chronic and long-term toxicities in patients with advanced cancer treated with ICIs [18]. Several studies have reported on earlier cessation of ICI in responding patients to prevent toxicities and to maintain a good functional status, considering that a significant portion of the patients could survive a long period or even be cured [19, 20].
Survivorship is a vital but often neglected part of cancer care. According to the National Coalition for Cancer Survivorship (NCCS), all patients with cancer are regarded as survivors from diagnosis to the end of life to as recognition of the potential ongoing problems of persons living with and beyond cancer and to emphasize the necessity to focus on unmet needs throughout the survivorship trajectory [21, 22]. Hence, we will use the term survivors for the rest of the manuscript. With the increasing rate of cancer diagnoses and treatment options, the number of cancer survivors is expected to increase continuously [23]. Recent estimates state that cancer survivors account for about 5% of the general population in Western countries [24].
Despite the significant body of evidence on survivorship outcomes, such as quality of life (QoL) and long-term toxicities, in survivors treated with chemotherapy, radiotherapy, and surgery, the evidence is limited in survivors treated with ICIs. Notwithstanding the remarkable rates of durable tumor response for some survivors, ICI treatments can be toxic. Most reports on immune-related adverse events (irAE) and the effects of irAEs on QoL are from clinical trials, reducing representativeness with real-world cohorts [25]. Additionally, most reports focused on the rates and risk factors of irAEs only instead of evaluating the effects of ICI toxicities on patient-reported outcomes [26, 27].
Recent observational studies involving real-world populations have reported on a range of outcomes related to survivorship, such as QoL, symptom burden, psychological distress, physical activity [28], and financial problems [29], and observed significant disruptions in several domains necessitating dedicated survivorship care in survivors treated with ICIs. However, the available studies varied in sample sizes, study designs, evaluated survivor outcomes, and survivor cohorts. With the increased indications and earlier use of ICIs, an expansion in the number of ICI-treated survivors is expected. Therefore, we systemically reviewed the survivorship outcomes faced by real-world cancer survivors treated with ICIs (as mono- or combination therapy). The scoping review methodology was selected considering the paucity of the quantitative evidence and heterogeneity of the studies. The aim of this scoping review was to evaluate the current status of the field and to create a research agenda by highlighting research gaps.
Methods
Literature search
We conducted a scoping review following the Joanna Briggs Institute (JBI) Methods Manual for Evidence Synthesis [30] and reported the results according to PRISMA Extension for Scoping Reviews (PRISMA-ScR) [31]. The study protocol was registered with the Open Science Framework (OSF) at the link https://osf.io/7eg5d. As suggested for scoping reviews, we used the PCC (Population/Concept/Context) instead of PICO: Population, survivors treated with ICIs; Concept, survivorship outcomes; Context, real-life setting [31]. We included studies specifically evaluating the survivorship outcomes in real-life context instead of reports from the clinical trials, in which the main focus was to evaluate the efficacy.
We used the Web of Science, PubMed, and Embase databases to systematically filter the published studies from January 1, 2010, until October 19, 2022. The selected MeSH search terms were as follows: (“health-related quality of life” OR “quality of life” OR “patient reported outcomes” OR “anxiety” OR “depression” OR “psychological” OR “fear of recurrence” OR “financial difficulty” OR “financial problem” OR “return to work” OR “late effect” OR “long-term immune-related adverse event” OR “survivorship” OR “survivor” OR “long-term survivor” OR “financial toxicity” OR “financial burden” OR “symptom burden” OR “psychological distress” OR “psychological well-being” OR “sexual”) AND (“immunotherapy” OR “immune checkpoint inhibitor”). The candidate search terms were selected with the help of a proposed survivorship care framework [32] by the research team. As the survivorship care framework used was very broad [32], we selected to prioritize the survivorship outcomes affecting the daily life of the survivors, and did not include the survivorship outcomes related to decision-making, caregiver burden, and health policy outcomes like coordination of the cancer care.
Inclusion and exclusion criteria
We included studies that met the following inclusion criteria: (1) prospective or retrospective study to investigate survivorship outcomes in survivors treated with ICI; (2) included a survivor cohort; (3) if survivors treated with targeted therapy and ICI were included, details about the ICI cohort were available; and (4) peer-reviewed full-text available in English. The exclusion criteria of studies were as follows: (1) review articles, case reports, case series, editorials, guidelines, dissertations, and opinion papers; (2) animal and cell-line studies; (3) studies including pediatric patients; (4) immunotherapy other than checkpoint inhibitors; (5) studies reporting on survival outcomes only without the data on the survivorship outcomes; (6) trial protocols; (7) studies evaluating the percentage and risk factors for individual adverse events only without an effect on patient-reported outcomes and late toxicities; (8) qualitative studies; and (9) clinical trials. We used the survivorship definition of the NCCS for the study inclusion and the discussion. In accordance with the NCCS definition, accepting all patients with cancer as cancer survivors after the cancer diagnosis, we included studies independent of the interval between the ICI start time and measurement of the survivorship outcomes.
Data extraction
Two authors (DCG, MST) extracted the data to an online data sheet following the PRISMA-ScR and JBI guidelines, and any discrepancy between the reviewers was resolved by the senior author (VA). For each study, lead author names, year of publication, study type, sample size, follow-up or evaluation time, tumor type, the ICI agent, evaluated survivorship outcomes, data collection methods, and main results were collected. If the included study evaluated more than one survivorship outcome (for example, both QoL and psychosocial problems), the data for all evaluated survivorship outcomes was extracted, and the individual survivorship outcomes from the included study were discussed in relevant subsections separately. Due to the heterogeneity of the study population and included studies, a narrative synthesis of data was performed. We did not evaluate the individual study qualities in line with the aims of scoping reviews and the recommendations of the JBI manual. Additionally, we searched on the clinicaltrials.gov website to screen ongoing studies evaluating the survivorship outcomes of survivors treated with ICIs.
Results
Study selection and descriptive characteristics of the studies
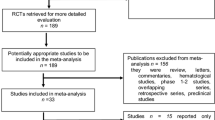
Our systematic search retrieved 20,056 records. After removing 6478 duplicates, we screened the remaining 13,578 records for inclusion. A total of 13,150 records were excluded after evaluating titles and abstracts. After evaluation of the full texts of the remaining 428 articles, we excluded a further 389 records as these studies evaluated other outcomes like efficacy only (n = 245), had no separate data for ICIs in the mixed cohorts (n = 67), evaluated combinations of ICIs with other immunotherapy agents (n = 10), used data from the databases or previously published datasets (n = 20), evaluated frequency and risk factors for individual irAEs only (n = 31), evaluated toxicities of radiotherapy in survivors treated with ICIs (n = 8), and qualitative studies (n = 8). A total of 39 studies from the systematic search were included in the review. The flowchart for article selection is shown in Fig. 1.
Melanoma was the most frequently evaluated tumor (n = 14), followed by non-small cell lung cancer (NSCLC) (n = 12). ICI monotherapy and ICI-ICI combinations were the most studied treatment regimes, while two studies included survivors treated with ICI plus chemotherapy regimens. Most studies were single center (n = 29) and small sample sizes of fewer than 50, while 17 studies had over 100 participants. Prospective cohorts (n = 14) and cross-sectional studies (n = 14) were the most prevalent study designs. The definition of survivor varied and mainly included predefined time points for ICI duration (6–12–24 months), while two studies only had survivors off therapy. QoL was the most commonly evaluated survivorship issue (n = 23), followed by toxicity burden (n = 16) and emotional problems (n = 9). Most studies were from the USA (n = 17), followed by China (n = 5) and Germany (n = 3). Pembrolizumab (n = 22) and nivolumab (n = 21) were the most frequently used ICIs in the available studies.
Assessment of QoL and functional status
A total of 23 studies evaluated QoL using validated questionnaires. The European Organisation for Research and Treatment of Cancer Core Quality of Life questionnaire (EORTC QLQ-C30) was used in 12 studies, followed by the Functional Assessment of Cancer Therapy (FACT) and EuroQol 5 dimensions 5 level (EuroQoL-5D-5L) in four studies each. The sample sizes varied between 18 and 412. Eleven studies were cross-sectional, four were retrospective, and eight were prospective (Table 1). The symptom and toxicity burden was evaluated with the symptom scales of the QoL questionnaires, semi-structured interviews, ECOG and Karnofsky performance status, and CTCAE grading of immune-related adverse events by the treating physician.
Most studies reported effects on the multiple QoL domains, and fatigue was the most frequently affected QoL domain, with fatigue rates of up to 90% reported with ICIs. However, a prospective study reported improved QoL with pembrolizumab monotherapy in melanoma survivors [33]. Furthermore, the rate of fatigue was very variable across the studies. While Mamoor et al. reported a 28% fatigue rate in melanoma survivors who were alive at least 12 months after treatment [26], the fatigue rate was 85% in a study including survivors with NSCLC without a time-prespecified treatment frame [34]. Two studies compared the QoL of the survivors treated with ICIs to the general population [34,35,36], and significantly worse QoL and functional well-being compared to the general population were noted in both studies. However, over 90% of the survivors with long-term disease control under ICIs were able to carry on routine daily activities. Similar to QoL, the outcomes regarding functional decline varied according to the evaluation time. Johnson et al. reported an ECOG status of 0 or 1 in 23 of the 24 patients with long-term survival with ICIs. The cohort’s median age was 60 years and most survivors returned to work [37]. In contrast, Singhal et al. reported a functional decline in 70% of the older adults (≥65 years) with lung cancer and recovery in only 13% of the survivors [38].
Factors associated with QoL
The factors affecting the QoL were evaluated in several studies. Jim et al. observed a similar QoL with different ICI regimens (all monotherapy) [34], while anti-PD-1 monotherapy was associated with better QoL compared to anti-PD-1/anti-CTLA-4 combination in survivors with melanoma in the study by Joseph et al. [33]. It should be noted that QoL was improved in survivors who responded to ICI therapy (mono- or in combination), while in survivors without a response to ICIs, QoL remained stable [33]. In addition to the ICI regimen, younger age and the need for subsequent therapy were associated with lower QoL in one study [39]. Schulz et al. observed that the QoL was lower in survivors who developed chronic and long-term irAEs than in survivors without these adverse events and similar QoL in patients with chronic autoimmune disorders and survivors with long-term and chronic irAEs [27]. In addition to these studies evaluating the denominators of lower QoL, two studies reported improvements in the QoL in survivors followed up with an electronic patient-reported outcome (ePRO) tool when compared with traditional care follow-up [40, 41].
Toxicity burden
The toxicity burden was most commonly evaluated as irAEs. Grade 3 or higher irAEs were observed in around 10–15% of the survivors, while in most studies, over 50% of the survivors had any grade irAEs. The prevalence of late irAEs was reported in three studies (Table 2). The definition of late and persistent irAEs in these studies differed from the recent Society for Immunotherapy of Cancer (STIC) recommendations for irAE terminology [53]. Nigro et al. defined late irAEs as irAEs occurred after >12 months of treatment and reported late irAEs in 30.1% of the survivors treated with at least 12 months of anti-PD-1/PD-L1 inhibitors [54]. Hall et al. reported irAEs in 24% of the survivors after at least 6 months of treatment and defined these irAEs as late irAEs [55]. Hsu et al. defined irAEs lasting over 12 months as long-term irAEs. They reported long-term irAEs in 23.7% of the survivors with non-small cell lung cancer treated with ICIs and late-onset irAEs (occurred at least 1 year after treatment initiation) in 16% of the patients [56]. In contrast, Argnani et al. evaluated 32 survivors with non-Hodgkin lymphoma and reported no late irAEs [57].
Psychological and neurocognitive problems
Eleven studies specifically evaluated psychological outcomes such as anxiety and depression with dedicated questionnaires (Table 3). All studies reported a high rate of anxiety and depression, ranging from 30 to 82%. While four studies cross-sectionally assessed anxiety and depression rates, two studies conducted longitudinal evaluations, and both reported decreasing levels of anxiety and depression during ICI treatment. The Hospital Anxiety and Depression Scale (HADS) was the most frequently used measurement (n = 5), followed by structured interviews and the distress thermometer. Sample sizes were mainly between 100 and 200, and almost all studies evaluated survivors with non-small cell lung cancer or melanoma. In addition to the prevalence of anxiety, depression, and psychological distress, two studies reported significantly lower overall response rates to ICIs in survivors with depression or psychological distress [49, 50]. Lastly, Thewes et al. reported fear of recurrence in 60% of the survivors with non-small cell lung cancer treated with ICIs, and another study with a limited sample size (73 survivors, of whom only nine were treated with ICIs) reported significantly higher fear of recurrence in adolescents and young adults treated with ICIs compared to survivors treated with other treatments [60].
Neurocognitive problems were addressed in four studies. Rogiers et al. evaluated neurocognitive function (NCF) in long-term survivors previously treated with pembrolizumab and ipilimumab and had their treatment ceased after long-term disease control [35]. The authors reported NCF disturbances, such as attention, memory, and executive function, in 32% and 41% of the survivors treated with pembrolizumab and ipilimumab, respectively. In addition to these problems, Invitto et al. reported an increasing rate of olfactory impairments and anosmia in elderly survivors treated with ICIs compared to survivors treated with CT or geriatric control subjects [61].
Abbreviations, Atezo, atezolizumab; BPRS, Brief Psychiatric Rating Scale; CTCAE, Common Terminology Criteria for Adverse Events; DCR, disease control rate; ECG, electrocardiogram; ECOG, Eastern Cooperative Oncology Group; EORTC QLQ-C30 PF, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Physical Functioning; FACT-M, Functional Assessment of Cancer Therapy Melanoma; HADS, Hospitalization Anxiety and Depression Scale; HRQoL, health-related quality of life; ICI, immune checkpoint inhibitor; Ipi, ipilimumab; irAE, immune-related adverse events; LSA, Life-Space Assessment; MoCA, Montreal Cognitive Assessment; NCCN-DT, National Comprehensive Cancer Network Distress Thermometer; NCF, neurocognitive function; Nivo, nivolumab; NPAE, neuropsychiatric adverse event; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; Pemb, pembrolizumab; PFS, progression-free survival; RCC, renal cell cancer; RCT, randomized controlled trial; STAI, State-Trait Anxiety Inventory
Financial toxicity
Financial toxicity was evaluated with a dedicated questionnaire (The Comprehensive Score for Financial Toxicity (COST)) in two studies. Thom et al. demonstrated that 23% of the survivors reported financial difficulties independent of sex, race, treatment type, treatment length, and status in 106 survivors with advanced melanoma [29]. In comparison, the risk of financial difficulties (p = 0.010) was higher in younger survivors (<65 years of age), and a higher percentage of younger survivors reported higher than expected out-of-pocket expenses due to treatment (14% vs. 2%). Only 14% of the younger and 10% of the older survivors reported satisfaction with their current financial situation. The presence of financial difficulties was moderately correlated with lower QoL.
McLouth et al. reported financial hardship in at least two domains in 52% of survivors with metastatic non-small cell lung cancer treated with CT or CT+ICIs [69]. Additionally, caregiver employment reduction was related to increased patient-reported financial hardship (68% vs. 40%) and financial distress. In addition to these studies, Lai-Kwon et al. reported EORTC QLQ-C30 financial domain results separately and observed financial difficulties in 20% of the survivors in their study conducted on 69 survivors with melanoma [44]. Johnson DB et al. reported that most long-term survivors previously treated with ipilimumab could return to work [37]. However, the exact return to work rate was unavailable in the study.
Other outcomes addressed
Patrinely et al. included 217 survivors with melanoma, renal cell carcinoma, and non-small cell lung cancer who survived over 2 years with ICI treatment and observed increased adiposity and muscle mass during treatment, while BMI, blood glucose, and blood pressure remained stable [39] (Table 4).
Ongoing studies
Our search on the clinicaltrials.gov website retrieved a total of 14 records. Almost all studies were active and in the recruiting phase. Sample sizes were variable and spanned less than a hundred participants to studies enrolling over 4000 survivors. Nine studies specifically cited immunotherapy survivorship, while the other five studies included both survivors on treatment with ICIs or basket cohorts including survivors treated with other treatment modalities in addition to ICIs. Three studies included melanoma survivors only, and the irAEs and QoL were the most commonly evaluated survivorship domains, similar to previously published studies (Supplementary Table).
Discussion
In this scoping review of 39 studies that included samples from real-life cohorts, we observed that QoL was impaired in survivors treated with ICIs. A significant portion of the survivors had delayed/late-onset and chronic adverse events and psychosocial problems in studies conducted in cohorts with prolonged survival with ICIs. However, most of the studies had limited sample sizes and limited follow-up, lacked an adequate control group, and did not follow the survivors longitudinally, pointing out a need for prospective studies with larger sample sizes. Additionally, several survivorship outcomes, like sexual problems, financial toxicity, and return to work, were very understudied, and further research is needed in these areas. To the best of our knowledge, our study is the first systematic review evaluating the survivorship outcomes in real-life cohorts.
In addition to the long-term burden of treatment toxicities and the effects of cancer on the body, cancer survivors are coping with psychological, physical, spiritual, and social problems [70]. Over 40% of cancer survivors have unmet survivorship needs, irrespective of treatment received [71, 72]. Furthermore, the survivors with unmet needs have a lower QoL, treatment satisfaction, and even survival [73]. Therefore, the comprehensive addressing of the survivorship issues, such as psychological problems and long-term toxicities, is paramount. However, the survivorship research with ICIs lagged, with QoL results mainly available from the clinical trials, necessitating the evaluation of survivorship in real-life cohorts. Haslam et al. estimated that around 40% of persons with cancer were eligible for treatment with ICIs according to FDA approvals as of 2019 [74]. Considering the rapid expansion of ICI indications in the last 3 years, these figures are expected to increase. Therefore, most survivors with advanced cancers and a sizeable portion of those with localized cancers treated with ICI could benefit from improvements in ICI survivorship care.
The irAEs are among the most important problems during treatment with ICIs. The frequency of all grade and severe irAEs was around 70 and 10% in the pivotal clinical trials [75,76,77]. The most frequent adverse events were skin and endocrine irAEs, while gastrointestinal and pulmonary irAEs were associated with higher morbidity and mortality [78]. The evidence from the real-life data confirmed these figures [79, 80]. Additionally, these observational studies demonstrated a high rate of late irAEs emerging after ICI cessation [81]. Further, these studies reported novel adverse events like carpal tunnel syndrome, uveitis, neurocognitive dysfunction, and chronic musculoskeletal problems [82,83,84]. However, most of these reports were case series focusing on the AE presentation and management. Further research is needed to define the prevalence of these possibly under-recognized adverse events as well as the effects of adverse events on survivors’ QoL, psychosocial problems, and functioning.
Psychosocial problems are frequent in patients with cancer, especially in survivors with advanced cancers. The available studies demonstrate a significant burden of psychosocial issues in survivors treated with ICIs [36, 66]. Even more, Rogiers et al. showed a 38% rate of anxiety and neurocognitive impairment in patients whose ICI treatments ceased after at least 12 months of disease control [35]. In addition to anxiety, depression, and sleep problems, qualitative studies evaluated the survivorship experience as a whole. They demonstrated additional complex problems like creating a new routine, issues with returning to activities of daily life, and constant feelings of uncertainty [85]. While the available studies used previously validated questionnaires like HADS to evaluate psychosocial problems [36, 60], lessons from qualitative studies point out a need to develop novel questionnaires to delineate these complex problems in survivors treated with ICIs due to the unique needs of these survivors with a lingering prognosis.
Several groups reported high rates of financial toxicity and out-of-pocket expenditures in ICI-treated survivors [29, 69]. At the same time, no study formally evaluated the return to work rates in ICI-treated survivors of working age. There is a wide discrepancy in financial toxicity across different countries due to differences across insurance policies and health economics, while all of the available studies regarding toxicity were from the USA. Therefore, reports of financial toxicity from countries with variable insurance coverage are needed. The importance of return to work is expected to increase due to the increased possibility of long-term disease control with the earlier use of ICIs in advanced cancers and the use of ICIs in the adjuvant/neoadjuvant setting and studies with large sample sizes, as well as the studies evaluating the reflections of work-related issues are required in ICI-treated patients.
Sexual problems are among the most overlooked problems in survivors treated with ICIs, with only a handful of studies with less than 40 survivors focusing only on male fertility [58]. However, considering the high rates of psychosocial problems in survivors treated with ICIs and high rates of sexual dysfunction in survivors with advanced cancers treated with chemotherapy or radiotherapy, a high rate of sexual problems could be expected for ICI-treated survivors [86, 87]. Furthermore, a recent preclinical study demonstrated decreased levels of ovarian follicles in an ICI-treated mice model [88]. The possibility of a similar female infertility problem in human studies, as well as studies evaluating the other dimensions of sexual dysfunction, is urgently needed.
The available literature highlights a need for higher quality studies in previously studied survivorship domains like QoL, toxicity, and psychosocial problems, as well as studies in understudied areas. As evident from the studies on QoL, the lack of control groups from the general population or patients treated with other treatments and the evaluation of survivorship outcomes in a single time point rather than longitudinal evaluation during the treatment were major problems in the available studies. Additionally, the unavailability of separate reporting for ICI-treated survivors in studies, including survivors treated with targeted therapy or chemotherapy in addition to ICIs, the lack of validated tools, short follow-up times in most studies, and limited sample sizes were other limitations. Prospective studies with larger sample sizes, control arms, and longitudinal follow-up are urgently needed. The longitudinal follow-up could aid in the understanding of changes in more problematic survivorship issues during the treatment course. For example, while acute irAEs could impact PROs earlier in the treatment course, work-related issues and limitations in the functional status could be more problematic in the long-term survivors.
Another critical point is the use of validated tools uniformly across studies to help extrapolate quantitative evidence and generate more reliable data in the form of meta-analyses in the future. Additionally, PRO assessments should be included in new studies evaluating ICI toxicities to delineate the effects of toxicities on the daily living of the survivors. As noted in the “Results” section of the review, the available studies did not comply with the recent irAE terminology recommendations by STIC. Later studies reporting according to these recommendations would increase the harmonization across studies and could aid in the extrapolation of the data more efficiently. Lastly, there is an urgent need for studies in understudied survivorship domains. These include but are not limited to financial toxicity, sexual problems, and work-related issues. With the expanded use of ICIs and the increase in the number of survivors in the advanced setting, more emphasis should be given to these survivorship outcomes, as evident in the review.
The main strength of this review is the comprehensive search strategy with the inclusion of several survivorship outcomes and the inclusive methodology. However, several limitations inherent to the scoping review methodology and to the included studies exist. First of all, most of the included studies had limited sample sizes and heterogeneous inclusion criteria. Albeit the broad search strategy in a scoping review methodology, the small number of identified quantitative studies limited our ability to discuss individual survivorship outcomes in more detail. The instruments to evaluate individual survivorship issues varied across the studies and this issue limited the cross-trial comparisons and quantitative synthesis of the data. Additionally, we did not filter the included studies according to study quality to be more inclusive. While this strategy was in accordance with the scoping review methodology, it limited the generalization of the results. Lastly, a significant portion of the studies evaluating the survivorship issues focused on the risk factors for irAEs instead of the effects of irAEs on PROs. Despite these limitations, the present review is the first systematic review evaluating the survivorship issues with ICIs in real-life cohorts and could serve as a guide to define the current status and delineate the areas needing further research to improve survivorship care in patients treated with ICIs.
Conclusion
In conclusion, the available evidence demonstrates that most survivors treated with ICIs have a significant toxicity burden, lower quality of life than the general population, and a high rate of psychosocial problems. Further research is needed to delineate the survivorship issues in survivors treated with ICIs in the adjuvant setting, in tumors other than melanoma and non-small cell lung cancer, as well as efforts to gather data on the understudied survivorship issues like fertility, financial toxicity, and return to work in survivors treated with ICIs.
References
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27.
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–203.
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2014;372(4):311–9.
Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9.
Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–45.
Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940.
Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30(6):507–19.
Lamba N, Ott PA, Iorgulescu JB. Use of first-line immune checkpoint inhibitors and association with overall survival among patients with metastatic melanoma in the anti–PD-1 era. JAMA Netw Open. 2022;5(8):e2225459–9.
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol. 2021;39(21):2339–49.
Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128(11):2085–97.
Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma: 5-year analysis of KEYNOTE-426. J Clin Oncol. 2023;41(17_suppl):LBA4501-LBA4501.
Eggermont AMM, Blank CU, Mandalà M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):643–54.
Gross ND, Miller DM, Khushalani NI, et al. Neoadjuvant cemiplimab for stage II to IV cutaneous squamous-cell carcinoma. N Engl J Med. 2022;387(17):1557–68.
Hu H, Kang L, Zhang J, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7(1):38–48.
Mollica MA, Smith AW, Tonorezos E, et al. Survivorship for individuals living with advanced and metastatic cancers: National Cancer Institute Meeting Report. J Natl Cancer Inst. 2022;114(4):489–95.
Sun L, Bleiberg B, Hwang W-T, et al. Association between duration of immunotherapy and overall survival in advanced non–small cell lung cancer. JAMA Oncol. 2023;9(8):1075–82.
Dimitriou F, Zaremba A, Allayous C, et al. Sustainable responses in metastatic melanoma patients with and without brain metastases after elective discontinuation of anti-PD1-based immunotherapy due to complete response. Eur J Cancer. 2021;149:37–48.
Twombly R. What's in a name: who is a cancer survivor? J Natl Cancer Inst. 2004;96(19):1414–5.
Shapiro CL. Cancer Survivorship. N Engl J Med. 2018;379(25):2438–50.
Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–505.
Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409–36.
Donia M, Kimper-Karl ML, Høyer KL, Bastholt L, Schmidt H, Svane IM. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur J Cancer. 2017;74:89–95.
Mamoor M, Postow MA, Lavery JA, et al. Quality of life in long-term survivors of advanced melanoma treated with checkpoint inhibitors. J Immunother Cancer. 2020;8(1)
Schulz TU, Zierold S, Sachse MM, et al. Persistent immune-related adverse events after cessation of checkpoint inhibitor therapy: prevalence and impact on patients' health-related quality of life. Eur J Cancer. 2022;176:88–99.
Valentine TR, Presley CJ, Carbone DP, Shields PG, Andersen BL. Illness perception profiles and psychological and physical symptoms in newly diagnosed advanced non-small cell lung cancer. Health Psychol. 2022;41(6):379–88.
Thom B, Mamoor M, Lavery JA, et al. The experience of financial toxicity among advanced melanoma patients treated with immunotherapy. J Psychosoc Oncol. 2021;39(2):285–93.
Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. J Natl Cancer Inst. 2019;111(11):1120–30.
Joseph RW, Liu FX, Shillington AC, et al. Health-related quality of life (QoL) in patients with advanced melanoma receiving immunotherapies in real-world clinical practice settings. Qual Life Res. 2020;29(10):2651–60.
Jim HSL, Eisel SL, Hoogland AI, Shaw S, King JC, Dicker AP. Use of a cancer registry to evaluate patient-reported outcomes of immune checkpoint inhibitors. Cancers (Basel). 2020;13(1)
Rogiers A, Leys C, Lauwyck J, et al. Neurocognitive function, psychosocial outcome, and health-related quality of life of the first-generation metastatic melanoma survivors treated with ipilimumab. J Immunol Res. 2020;2020:2192480.
Rogiers A, Leys C, De Cremer J, et al. Health-related quality of life, emotional burden, and neurocognitive function in the first generation of metastatic melanoma survivors treated with pembrolizumab: a longitudinal pilot study. Support Care Cancer. 2020;28(7):3267–78.
Johnson DB, Friedman DL, Berry E, et al. Survivorship in immune therapy: assessing chronic immune toxicities, health outcomes, and functional status among long-term ipilimumab survivors at a single referral center. Cancer Immunol Res. 2015;3(5):464–9.
Singhal S, Walter LC, Smith AK, et al. Change in four measures of physical function among older adults during lung cancer treatment: a mixed methods cohort study. J Geriatr Oncol. 2022;
Patrinely JR Jr, Young AC, Quach H, et al. Survivorship in immune therapy: assessing toxicities, body composition and health-related quality of life among long-term survivors treated with antibodies to programmed death-1 receptor and its ligand. Eur J Cancer. 2020;135:211–20.
Tolstrup LK, Pappot H, Bastholt L, Möller S, Dieperink KB. Impact of patient-reported outcomes on symptom monitoring during treatment with checkpoint inhibitors: health-related quality of life among melanoma patients in a randomized controlled trial. J Patient Rep Outcomes. 2022;6(1):8.
Zhang L, Zhang X, Shen L, Zhu D, Ma S, Cong L. Efficiency of electronic health record assessment of patient-reported outcomes after cancer immunotherapy: a randomized clinical trial. JAMA Netw Open. 2022;5(3):e224427–7.
Iivanainen S, Alanko T, Peltola K, et al. ePROs in the follow-up of cancer patients treated with immune checkpoint inhibitors: a retrospective study. J Cancer Res Clin Oncol. 2019;145(3):765–74.
Bergerot CD, Bergerot PG, Philip EJ, et al. Perception of cure among patients with metastatic genitourinary cancer initiating immunotherapy. J Immunother Cancer. 2019;7(1):71.
Lai-Kwon J, Khoo C, Lo S, et al. The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors. J Cancer Surviv. 2019;13(4):503–11.
Hyatt A, Drosdowsky A, Williams N, et al. Exercise behaviors and fatigue in patients receiving immunotherapy for advanced melanoma: a cross-sectional survey via social media. Integr Cancer Ther. 2019;18:1534735419864431.
Steffen McLouth LE, Lycan TW, Levine BJ, et al. Patient-reported outcomes from patients receiving immunotherapy or chemoimmunotherapy for metastatic non–small-cell lung cancer in clinical practice. Clin Lung Cancer. 2020;21(3):255–263.e254.
O'Reilly A, Hughes P, Mann J, et al. An immunotherapy survivor population: health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support Care Cancer. 2020;28(2):561–70.
Yin X, Cheng Y, Yao S, et al. Fear of cancer recurrence is related to the efficacy of immunotherapy and quality of life in patients with NSCLC during the COVID-19 pandemic in China. Am J Cancer Res. 2022;12(8):4040–9.
Li W, Bi Z, Wu J, et al. Effect of depression disorder on the efficacy and quality of life of first-line chemotherapy combined with immunotherapy in oncogene-driver negative NSCLC patients. Front Oncol. 2022;12:772102.
Bi Z, Li W, Zhao J, et al. Negative correlations of psychological distress with quality of life and immunotherapy efficacy in patients with advanced NSCLC. Am J Cancer Res. 2022;12(2):805–15.
Koldenhof JJ, van der Baan FH, Verberne EG, et al. Patient-reported outcomes during checkpoint inhibition: insight into symptom burden in daily clinical practice. J Pain Symptom Manage. 2022;63(6):997–1005.
Wong ML, Shi Y, Smith AK, et al. Changes in older adults’ life space during lung cancer treatment: a mixed methods cohort study. J Am Geriatr Soc. 2022;70(1):136–49.
Jarushka N, Catherine M, Michael BA, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J Immunother Cancer. 2023;11(3):e006398.
Nigro O, Pinotti G, De Galitiis F, et al. Late immune-related adverse events in long-term responders to PD-1/PD-L1 checkpoint inhibitors: a multicentre study. Eur J Cancer. 2020;134:19–28.
Hall KH, Liu Y, Jiang C, Harvey RD. New and worsening long-term immune-related adverse events with PD-1/PD-L1 pathway agents in patients with cancer. pharmacotherapy: The Journal of Human Pharmacology and Drug. Therapy. 2020;40(2):133–41.
Hsu ML, Murray JC, Psoter KJ, et al. Clinical Features, survival, and burden of toxicities in survivors more than one year after lung cancer immunotherapy. Oncologist. 2022;27(11):971–81.
Argnani L, Casadei B, Pelusi C, et al. Immune-related adverse events in the treatment of non-Hodgkin lymphoma with immune checkpoint inhibitors. Sci Rep. 2022;12(1):1753.
Salzmann M, Tosev G, Heck M, et al. Male fertility during and after immune checkpoint inhibitor therapy: a cross-sectional pilot study. Eur J Cancer. 2021;152:41–8.
Tong J, Kartolo A, Yeung C, Hopman W, Baetz T. Long-term toxicities of immune checkpoint inhibitor (ICI) in melanoma patients. Curr Oncol. 2022;29(10):7953–63.
Thewes B, Kaal SEJ, Custers JAE, et al. Prevalence and correlates of high fear of cancer recurrence in late adolescents and young adults consulting a specialist adolescent and young adult (AYA) cancer service. Support Care Cancer. 2018;26(5):1479–87.
Invitto S, Leucci M, Accogli G, et al. Chemobrain, olfactory and lifestyle assessment in onco-geriatrics: sex-mediated differences between chemotherapy and immunotherapy. Brain Sci. 2022;12(10)
Kovács P, Pánczél G, Borbola K, Juhász G, Liszkay G. Psychological Changes in melanoma patients during ipilimumab treatment compared to low-dose interferon alpha therapy—a follow-up study of first experiences. Pathol Oncol Res. 2014;20(4):939–44.
McFarland DC. New lung cancer treatments (immunotherapy and targeted therapies) and their associations with depression and other psychological side effects as compared to chemotherapy. Gen Hosp Psychiatry. 2019;60:148–55.
Wiens L, Schäffeler N, Eigentler T, Garbe C, Forschner A. Psychological distress of metastatic melanoma patients during treatment with immune checkpoint inhibitors: results of a prospective study. Cancers (Basel). 2021;13(11)
Bodd MH, Locke SC, Wolf SP, et al. Patient-reported distress and clinical outcomes with immuno-oncology agents in metastatic non-small cell lung cancer (mNSCLC): a real-world retrospective cohort study. Lung Cancer. 2022;175:17–26.
Andersen BL, McElroy JP, Carbone DP, et al. Psychological symptom trajectories and non-small cell lung cancer survival: a joint model analysis. Psychosom Med. 2022;84(2):215–23.
Xie Q, Sun C, Fei Z, Yang X. Accepting immunotherapy after multiline treatment failure: an exploration of the anxiety and depression in patients with advanced cancer experience. Patient Prefer Adherence. 2022;16:1–9.
Khalil RB, Kassab A, Costa G, Haddad FG, Richa S, Kattan J. Increased psychomotor retardation and tension as short-term neuropsychiatric adverse events of immune checkpoint inhibitors: a prospective cohort study. J Cancer Res Ther. 2022;18(1):140–6.
McLouth LE, Nightingale CL, Levine BJ, et al. Unmet care needs and financial hardship in patients with metastatic non-small-cell lung cancer on immunotherapy or chemoimmunotherapy in clinical practice. JCO Oncol Pract. 2021;17(8):e1110–9.
Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 Suppl):2577–92.
Batehup L, Gage H, Williams P, et al. Unmet supportive care needs of breast, colorectal and testicular cancer survivors in the first 8 months post primary treatment: a prospective longitudinal survey. Eur J Cancer Care (Engl). 2021;30(6):e13499.
Mlakar I, Lin S, Aleksandraviča I, et al. Patients-centered SurvivorShIp care plan after Cancer treatments based on Big Data and Artificial Intelligence technologies (PERSIST): a multicenter study protocol to evaluate efficacy of digital tools supporting cancer survivors. BMC Med Inform Decis Mak. 2021;21(1):243.
Chen M, Li R, Chen Y, et al. Unmet supportive care needs and associated factors: evidence from 4195 cancer survivors in Shanghai. China Front Oncol. 2022;12:1054885.
Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3(3):e200423.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26.
Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–80.
Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254–67.
Abbas W, Aggarwal A, Pankaj P, Jain R. Real-world data of second-line immunotherapy in metastatic clear cell renal cell carcinoma: a retrospective study. Cancer Res Stat Treat. 2021;4(1):55–60.
Miao K, Zhang X, Wang H, et al. Real-world data of different immune checkpoint inhibitors for non-small cell lung cancer in China. Front Oncol. 2022;12
Owen CN, Bai X, Quah T, et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann Oncol. 2021;32(7):917–25.
Lechevalier D, Denis D, Le Corre Y, et al. Carpal Tunnel syndrome: a new adverse effect of immune checkpoint inhibitors, 11 Cases. J Immunother. 2021;44(3):122–6.
Fardeau C, Bencheqroun M, Levy A, et al. Uveitis associated with cancer immunotherapy: long-term outcomes. Immunotherapy. 2021;13(18):1465–81.
Ng AH, Molinares DM, Guo Y, Fu J, Bruera E. Functional impairments and rehabilitation outcomes of patients with immunotherapy-induced acute inflammatory demyelinating polyradiculoneuropathy, myasthenia gravis, and myositis. Am J Phys Med Rehabil. 2021;100(10):1015–9.
Kamminga NCW, van der Veldt AAM, Joosen MCW, et al. Experiences of resuming life after immunotherapy and associated survivorship care needs: a qualitative study among patients with metastatic melanoma*. Br J Dermatol. 2022;187(3):381–91.
Sousa Rodrigues Guedes T, Barbosa Otoni Gonçalves Guedes M, de Castro Santana R, et al. Sexual dysfunction in women with cancer: a systematic review of longitudinal studies. Int J Environ Res Public Health. 2022;19(19):11921.
Barcellini A, Dominoni M, Dal Mas F, et al. Sexual health dysfunction after radiotherapy for gynecological cancer: role of physical rehabilitation including pelvic floor muscle training. Front Med. 2022;8
Xu PC, Luan Y, Yu SY, Xu J, Coulter DW, Kim SY. Effects of PD-1 blockade on ovarian follicles in a prepubertal female mouse. J Endocrinol. 2021;252(1):15–30.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
V.A. and MSY.T. created the study question. V.A., MSY.T., and D.C.G. designed the study. D.C.G. and MSY.T. conducted the initial systematic search. D.C.G. wrote the first draft of the manuscript and the tables. MSY.T. and V.A. conducted the supervising and editing of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Deniz Can Güven is a visiting scholar.
Supplementary information
ESM 1
(DOCX 20 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Güven, D.C., Thong, M.S. & Arndt, V. Survivorship outcomes in patients treated with immune checkpoint inhibitors: a scoping review. J Cancer Surviv (2024). https://doi.org/10.1007/s11764-023-01507-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-023-01507-w