Abstract
Purpose
The aim of this study was to assess the evolution of health-related quality of Life (HRQoL), emotional burden, and neurocognitive function in the first-generation metastatic melanoma survivors treated with pembrolizumab.
Methods
Survivors were defined as patients who achieved a durable remission for at least 6 months after initiating pembrolizumab in a single-center observational study (N = 141). A semi-structured interview was performed at baseline. Neurocognitive computerized testing and patient-reported outcomes were collected at 4 time points to assess HRQoL using the EORTC QLQ-C30 and the HADS to assess anxiety and depression.
Results
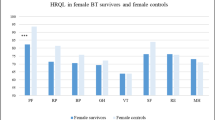
Out of 35 eligible patients, 25 were recruited and completed baseline assessment (18 female; median age 58 years [range 28–86]; 24 completed the 1-year follow-up phase. Median time since diagnosis was 30 months (range 12–84); median time since initiation of pembrolizumab was 19 months (range 6–42). At all visits, survivors reported a significantly lower global HRQoL, lower physical, emotional, cognitive, role, and social functioning compared with the European Mean of the healthy population. Fifteen patients (64%) had clinical levels of anxiety/depression at one time point during follow-up. The clinical interview revealed that 12 patients (48%) suffered from Cancer-Related-Post-Traumatic-Stress disorder, of whom 7 (28%) developed transient suicidal ideation, 1 patient made a suicide attempt. Neurocognitive testing revealed cognitive impairment in 8 patients (32%).
Conclusions
Metastatic melanoma survivors, treated successfully with pembrolizumab, are at risk for suffering from emotional distress and neurocognitive impairment with a persistent impact on their HRQOL. Timely detection in order to offer tailored care is indicated.



Similar content being viewed by others
Data availability
Data and material is available upon request from the corresponding author
References
Liu L, O’Donnell P, Sullivan R, Katalinic A, Moser L, de Boer A et al (2016) Cancer in Europe: death sentence or life sentence? Eur J Cancer 65:150–155
Annunziata MA, Muzzatti B, Flaiban C, Gipponi K, Carnaghi C, Tralongo P, Caruso M, Cavina R, Tirelli U (2018) Long-term quality of life profile in oncology: a comparison between cancer survivors and the general population. Support Care Cancer 26:651–656
Paltrinieri S, Fugazzaro S, Bertozzi L, Bassi MC, Pellegrini M, Vicentini M, Mazzini E, Costi S (2018) Return to work in European Cancer survivors: a systematic review. Support Care Cancer 26:2983–2994
Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejbergs L et al (2019) Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol
Rogiers A, Boekhout A, Schwarze JK, Awada G, Blank CU, Neyns B (2019) Long-term survival, quality of life, and psychosocial outcomes in advanced melanoma patients treated with immune checkpoint inhibitors. J Oncol 2019:5269062
Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, Hwu WJ, Weber JS, Gangadhar TC, Joseph RW, Dronca R, Patnaik A, Zarour H, Kefford R, Hersey P, Zhang J, Anderson J, Diede SJ, Ebbinghaus S, Hodi FS (2018) Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol 36:1668–1674
Schadendorf D, Dummer R, Hauschild A, Robert C, Hamid O, Daud A, van den Eertwegh A, Cranmer L, O'Day S, Puzanov I, Schachter J, Blank C, Salama A, Loquai C, Mehnert JM, Hille D, Ebbinghaus S, Kang SP, Zhou W, Ribas A (2016) Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer 67:46–54
Langbaum T, Smith TJ (2019) Time to study metastatic-cancer survivorship. N Engl J Med 380:1300–1302
O’Reilly A, Hughes P, Mann J, Lai Z, Teh JJ, McLean E, et al. (2019) An immunotherapy survivor population: health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support Care Cancer https://doi.org/10.1007/s00520-019-04818-w
Hamel JF, Pe M, Coens C, Martinelli F, Eggermont AM, Brandberg Y, Bottomley A (2016) A systematic review examining factors influencing health related quality of life among melanoma cancer survivors. Eur J Cancer 69:189–198
Beutel ME, Fischbeck S, Binder H, Blettner M, Brahler E, Emrich K et al (2015) Depression, anxiety and quality of life in long-term survivors of malignant melanoma: a register-based cohort study. PLoS One 10:e0116440
Rogiers A, Boekhout A, Schwarze JK, Awada G, Blank CU, Neyns B (2019) Long-term survival, quality of life, and psychosocial outcomes in advanced melanoma patients treated with immune checkpoint inhibitors. J Oncol 1-17 https://doi.org/10.1155/2019/5269062
Levy D, Dhillon HM, Lomax A, Marthick M, McNeil C, Kao S, et al. (2018) Certainty within uncertainty: a qualitative study of the experience of metastatic melanoma patients undergoing pembrolizumab immunotherapy. Support Care Cancerhttps://doi.org/10.1007/s00520-018-4443-3
Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA (2014) A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother 63:1115–1128
Rosenblat JD, Cha DS, Mansur RB, McIntyre RS (2014) Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuro-Psychopharmacol Biol Psychiatry 53:23–34
Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S et al (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:200
Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR (2008) Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 26:971–982
Walker AK, Martelli D, Ziegler AI, Lambert GW, Phillips SE, Hill SJ, McAllen R, Sloan EK (2019) Circulating epinephrine is not required for chronic stress to enhance metastasis. Psychoneuroendocrinology. 99:191–195
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM (1997) A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med 27:363–370
Bocerean C, Dupret E (2014) A validation study of the Hospital Anxiety and Depression Scale (HADS) in a large sample of French employees. BMC Psychiatry 14:354
Castelli L, Binaschi L, Caldera P, Mussa A, Torta R (2011) Fast screening of depression in cancer patients: the effectiveness of the HADS. Eur J Cancer Care (Engl) 20:528–533
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
EORTC B (2017) EORTC Quality of life group translation procedure, fourth edn. EORTC, Brussels, p 24
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121–1123
Stone P, Richards M, A’Hern R, Hardy J (2000) A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol 11:561–567
Rietberg MB, Van Wegen EE, Kwakkel G (2010) Measuring fatigue in patients with multiple sclerosis: reproducibility, responsiveness and concurrent validity of three Dutch self-report questionnaires. Disabil Rehabil 32:1870–1876
Debouverie M, Pittion S, Guillemin F, Vespignani H (2002) Fatigue scales used in multiple sclerosis. Rev Neurol (Paris) 158:1139–1143
Caine C, Deshmukh S, Gondi V, Mehta M, Tome W, Corn BW et al (2016) CogState computerized memory tests in patients with brain metastases: secondary endpoint results of NRG Oncology RTOG 0933. J Neuro-Oncol 126:327–336
Vardy J, Wong K, Yi QL, Park A, Maruff P, Wagner L, Tannock IF (2006) Assessing cognitive function in cancer patients. Support Care Cancer 14:1111–1118
Cogstate L (2017) Cogstate pediatric and adult normative data. Copyright © 2017 CogState, Ltd. All rights reserved.
Cromer JA, Schembri AJ, Harel BT, Maruff P (2015) The nature and rate of cognitive maturation from late childhood to adulthood. Front Psychol 6:704
Hammers D, Spurgeon E, Ryan K, Persad C, Barbas N, Heidebrink J, Darby D, Giordani B (2012) Validity of a brief computerized cognitive screening test in dementia. J Geriatr Psychiatry Neurol 25:89–99
Yoshida T, Suga M, Arima K, Muranaka Y, Tanaka T, Eguchi S et al (2011) Criterion and construct validity of the CogState Schizophrenia Battery in Japanese patients with schizophrenia. PLoS One 6:e20469
Ingraham (1996) An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology 10:120–124
Wefel JS, Vardy J, Ahles T, Schagen SB (2011) International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 12:703–708
Ventura J, Liberman RP, Green MF, Shaner A, Mintz J (1998) Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P). Psychiatry Res 79:163–173
Hinz A, Singer S, Brahler E (2014) European reference values for the quality of life questionnaire EORTC QLQ-C30: results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol 53:958–965
Kasparian NA, McLoone JK, Butow PN (2009) Psychological responses and coping strategies among patients with malignant melanoma: a systematic review of the literature. Arch Dermatol 145:1415–1427
Shalev A, Liberzon I, Marmar C (2017) Post-traumatic stress disorder. N Engl J Med 376:2459–2469
Lai-Kwon J, Khoo C, Lo S, Milne D, Mohamed M, Raleigh J, et al. (2019) The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors. J Cancer Surviv
Dieng M, Butow PN, Costa DS, Morton RL, Menzies SW, Mireskandari S, Tesson S, Mann GJ, Cust AE, Kasparian NA (2016) Psychoeducational intervention to reduce fear of cancer recurrence in people at high risk of developing another primary melanoma: results of a randomized controlled trial. J Clin Oncol 34:4405–4414
Bartels F, Stronisch T, Farmer K, Rentzsch K, Kiecker F, Finke C (2019) Neuronal autoantibodies associated with cognitive impairment in melanoma patients. Ann Oncol https://doi.org/10.1093/annonc/mdz083
Duncan M, Moschopoulou E, Herrington E, Deane J, Roylance R, Jones L et al (2017) Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open 7:e015860
Conklin HM, Ogg RJ, Ashford JM, Scoggins MA, Zou P, Clark KN, Martin-Elbahesh K, Hardy KK, Merchant TE, Jeha S, Huang L, Zhang H (2015) Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. J Clin Oncol 33:3894–3902
Acknowledgments
We would like to thank the patients and their family for participating in this study, and Yanina Jansen, Julia K. Schwarze, and Laila Ben Salama for helping with the data collection. We thank Prof Paul Maruff, Chief Science Officer of Cogstate Ltd and Associate Professor at the Florey Institute for Neuroscience and Mental Health, for the critical reading and his constructive input that helped us to improve the manuscript.
Author information
Authors and Affiliations
Contributions
A. Rogiers conceived and performed the study design, performed the data collection, developed the manuscript, the data analysis, and the data interpretation; Christophe Leys commented on the manuscript, performed the data analysis, the statistical analysis, and took part in the interpretation of the data; Jennifer De Cremer assisted on the data collection and commented on the manuscript. Gil Awada assisted on the data collection and commented on the manuscript. Adrian Schembri assisted in the study design, commented on the manuscript, and assisted to the data analysis; Peter Theuns commented on the manuscript; Mark de Ridder commented on the manuscript; Bart Neyns assisted in the study design, took part in the data interpretation, and developed and commented the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study (ClinicalTrials.gov Identifier: NCT02673970) on “Biomarkers for the activity of immune checkpoint inhibitor therapy in patients with advanced melanoma”, was approved by the Ethical Committee (EC) of the Universitair Ziekenhuis Brussel in 2014, and the sub-study in April 2016.
Conflict of interest
AR has consulting and advisory role in the Bristol-Myers Squibb and Merck Sharp & Dome. GA received travel accommodations from the Merck Sharp & Dome and Pfizer, research grant from the Pfizer and Novartis. AS is a full-time employee of the Cogstate Ltd., the company that provided the computerized cognitive tests in this study. MD received research agreement from the Brainlab AG, and has consulting or advisory role in the Novalis Certification Expert. BN received honoraria from the Bristol-Myers Squibb, Merck Sharp & Dome, Novartis, and Roche, has consulting or advisory role in the Bristol-Myers Squibb, Merck Sharp & Dome, Novartis, Roche, Speakers’ Bureau-Novartis, and received travel, accommodations, and expenses from the Amgen, Bristol-Myers Squibb, Merck Sharp & Dome, Novartis, and Roche.The other authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rogiers, A., Leys, C., De Cremer, J. et al. Health-related quality of life, emotional burden, and neurocognitive function in the first generation of metastatic melanoma survivors treated with pembrolizumab: a longitudinal pilot study. Support Care Cancer 28, 3267–3278 (2020). https://doi.org/10.1007/s00520-019-05168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05168-3