Abstract
More than 20 years after remedial measures were carried out, six plots (Š1 – Š6) at the Šobov locality were examined in more detail. From a pedological viewpoint, the physico-chemical differences of the soils in these areas are not the result of pedogenesis. This is the effect of extremely acidic mineralised solutions that have leaked from or are still leaking from the heap at the site. The plant community here is most often poor to weakly developed with acidophilic vegetation (Š1, Š2, Š5), without vegetation (Š4) or with dense species-rich vegetation (Š3, Š6). Saprotrophic microscopic fungi of the phylum Zygomycota are notably suppressed and their diversity is low. Species of the genera Absidia and Zygorhynchus were found most often. In contrast, the diversity of the phylum Ascomycota is notably high. The genera Penicillium (35 species), Aspergillus (7 species) and Trichoderma (5 species) are dominantly represented. Species of microscopic filamentous fungi in every soil sample that did not occur in the other samples were also recorded; i.e. they form the specific soil mycobiome of the given location. From the 15 types of keratinophilic fungi, the most commonly occurring were Purpureocillium lilacinum and Keithomyces carneus. Keratinolytic properties were recorded only in the species Trichophyton ajelloi. Soil reaction is the most important ecological factor that influences the biological properties of soils.
Similar content being viewed by others
Introduction
Banská Štiavnica was the most significant mining town in Slovakia. As early as the beginning of the thirteenth century, gold, silver and precious metal minerals containing non-ferrous metals such as lead, zinc and copper were mined here and gradually became the main object of mining. A part of Banská Štiavnica is the locality Šobov, which since 1999 has been registered as an area of environmental burden. On the site is a heap of unsorted mining waste from secondary quartz rock that was used to make “dinas” type bricks. Rainwater, upon entering the heap, seeped through the pyrite deposits and formed sulphuric acid, which in turn increased the migration of toxic elements originally embedded in the crystal structure of pyrite and destabilised the structure of clay minerals, releasing toxic Al3+ cations. The chemical weathering of sulphides, supported the whole time by geochemical and microbiological reactions, enabled contaminants to mobilise and enter the hydrological cycle. Quartz rock mining ceased here in the 1990s after 50 years of continuous operation. Subsequently, in the years 1995–1998, the companies DINAS, a. s., and BAPA, s. r. o., started taking remedial measures to minimise the threat to the environment. In 1999, a system of pools intended to maintain and decontaminate the acid mine drainage (AMD) was built and was supposed to prevent the spread of contamination below the heap. The first such basin was designed as an anoxic limestone drainage (ALD), the second as an anaerobic pond, and the third as the aerobic part of the system planted with narrowleaf cattail (Nosalj et al. 2021). However, even after this remediation, previous studies (Čaja and Zima 2014; Madaras et al. 1999) have confirmed significant changes in the soils and their chemical properties, which also affected soil mycocenosis (Adamcová 2010; Adamcová et al. 2012; Nosalj et al. 2021; Šimonovičová et al. 2019).
The aim of this contribution is, more than 20 years after the remediation measures were implemented, to specify in more detail the individual soil types that have been affected long-term by acidification, their physico-chemical properties and the plant community growing on them and to characterise and compare soil mycocenosis, including the keratinophilic species from the original three areas, at six areas at the Šobov locality. The individual areas differ in varieties of soil type, chemical composition as well as content of reactive aluminium and iron, type of vegetation and rehabilitation interventions.
Determination of kertinophilic fungi as a part of the soil mycocenosis is of great importance as they play a significant role in the decomposition and therefore their presence may indicate the effect of acidification on the level of decomposition processes in affected soils.
Material and methods
Research plots at the Šobov locality
Fifty years of continuous mining of quartz rock at the Šobov location had a significant impact on the ecosystem of the surrounding environment. A channel was artificially created for the AMD drainage (Fig. 1a). The AMD also flowed along the slope, where a strongly acidified and eroded part was gradually formed on one side, with a less damaged part with acid-loving grasses on the other side (Fig. 1b). The eroded surface in the lower part of the slope gradually became overgrown with mosses (Fig. 1c). Within the rehabilitation and reclamation measures, a system of pools (Fig. 2a, b, c) was built below the heap for AMD decontamination. The eroded parts of the locality gradually began to be grown over.
Artificially created channel for the AMD drainage (a), AMD flowing down the slope, which divided the area into an area heavily damaged by acidification (no vegetation) and a less damaged area (acidophilic grasses) (b), an eroded area gradually overgrown with mosses (c). Figures are from the end of 90-is years of twentieth century
For this study, we selected 6 plots at the Šobov location (Fig. 3), which differ from one another in several parameters. Plots Š1 – Š3 and Š6 are located on the part of the slope where rehabilitation measures were applied and where mowing takes place at least once a year.
Plot Š1 (GPS coordinates S 48°28′20.80" E 18°54′25.48"). Despite the mentioned measures, this plot is affected by a high measure of water erosion; it is without vegetation cover or has only isolated tufts of grass (Fig. 4a). Plot Š2 (GPS coordinates S 48°28′20.04" E 18°54′25.23") is located about 50 m from the previous plot Š2 and is covered by scattered acidophilic vegetation (Fig. 4b). Plot Š3 (GPS coordinates S 48°28′19.42" E 18°54′24.96") is located about 50 m from the previous plot Š3 and the vegetation cover here is compact (Fig. 4c). Plot Š4 (GPS coordinates S 48°28′21.09" E 18°54′21.35") is located above M. M. Hodžu Road near the pool that collects the acid mine waters (AMD) from below the heap. This plot is outside the rehabilitated area (Fig. 5a). Plot Š5 (GPS coordinates S 48°28′22.57" E 18°54′28.02") is located near plot Š1 and is part of the slope that was divided by the flowing AMD below the heap prior to the pool being built. This part of the slope has not yet been rehabilitated and is heavily damaged by water erosion (Fig. 5b). Plot Š6 (GPS coordinates N 48°28′10.53" E 18°54′19.08") is an area almost at the end of the meadow vegetation in the direction of plots Š1, Š2 and Š3, at a distance of approx. 200 m from Š3 with a fully connected diverse meadow cover (Fig. 5c). All the soil types were classified according to the World Reference Base for Soil Resources (IUSS Working Group WRB 2022).
Research plots at the Šobov locality: Š1 – affected by contamination, where the absence of vegetation cover in the past caused erosion; at present it is covered with vegetation only sporadically (a), Š2 – significant reduction of the most damaged plot (b), Š3 – meadow growth with acidophilic vegetation (c)
Soil sampling and basic chemical analyses
At each plot (Š1 – Š6), we took samples from the topsoil to a depth of 15 cm, always at five sampling points, and put them in plastic bags with the location marked on the front. The average soil sample thus always contained a mixture of soil from all five sampling points at each plot. Under laboratory conditions, we homogenised the soil samples, then quartered them and modified them into a fine soil by sieving them through a sieve with 2 mm holes. We stored the soil samples prepared in this way in a refrigerator at 4 °C in the dark and then used them for all chemical and mycological analyses.
We determined the soil reaction (pH in H2O), total carbon (%) and total nitrogen (%) in the soil samples. The value of soil organic matter (SOM) content was calculated from %Corg multiplied by a conversion factor of 1.724 (Hrivňáková et al. 2011). We used the EA-TCD method for carbon and nitrogen analyses and determined reactive Al (mg/kg) and reactive Fe (mg/kg) using the ICP-AES method. All analyses were carried out at the accredited Central Forestry Laboratory in Zvolen.
Plant community structure
Vegetation on the plots was sampled on 10 June 2023 according to the Zurich-Montpellier school (Braun-Blanquet 1964) using the 7-item Braun-Blanquet scale. Nomenclature of non-vascular and vascular plants follows Marhold and Hindák (1998).
Mycological analyses
The presence of cultivable mycobiota was investigated by the conventional dilution plating method, as described in previous reports by Adamcová et al. (2012), Nosalj et al. (2021) and Šimonovičová et al. (2019). Isolation of so-called heat-resistant fungi was described in a study by Adamcová et al. (2012).
The occurrence and diversity of so-called keratinophilic fungi (having an affinity towards keratinous substrata) were determined using the hair baiting method, as described by Javoreková et al. (2012) with the following modification. From each soil sample taken on the given plot, we weighed 5 g of soil and 0.5 g of vermiculite into 10 Petri dishes with a diameter of 55 mm (i.e. 10 subsamples per sample) and then moistened the samples with 5 mL of chloramphenicol solution (100 mg/L) and cycloheximide (500 mg/L). We then put 10 pieces of sterile degreased horse hair, 2 to 3 cm long, on the surface of the samples and incubated the samples for one month at a laboratory temperature of 25 °C. We then inoculated the microscopic fungi that were found on the hair with MEA (Malt Extract Agar) and SDA (Saboraud Dextrose Agar, both Himedia Mumbai, India) nutrient media. We identified the species both phenotypically (de Hoog et al. 2020) and using the PCR method. We performed DNA extraction using the DNeasy Plant Minikit (Qiagen, Hilden, Germany). Phylogenetically informative sequences were obtained from the region of the internal transcribed spacer (ITS) and the nuclear large subunit (LSU) of the rDNA. Regions of internal transcribed spacer (ITS) were amplified using primer pair ITS1 and ITS4. All reactions were performed in an Eppendorf Gradient MasterCycler (Eppendorf,Hamburg). Conditions for amplification of ITS: 95 °C for 2 min and 30 s (initial denat-uration), 35 cycles of 94 °C for 30 s (denaturation), 54 °C for 30 s (annealing), 72 °C for 1 min (elongation), and 72 °C for 5 min (final elongation). The PCR purification was performed using a Monarch PCR & DNA Cleanup kit (5 μg) (New England Biolabs, USA). Sanger sequencing was performed by LGC Genomics (Berlin, Germany) (Labuda et al. 2021).
Results and discussion
Characteristic of Soils
The soil cover at the Šobov research area was originally formed by cambic modals, which developed from andesite tuffs and quartzites. The soil reaction of the soils was originally weakly acidic (pH 6.1) with a relatively high content of accessible basic cations and a saturation of the sorption complex of up to 82%. This was originally arable land which is now permanently covered in grass and is occasionally mowed. We can classify it as cambic cultivation soil, with different varieties according to the degree and consequences of soil contamination by the extremely acidic waters flowing from the heap. An accompanying sign is also the lack of vegetation cover in some places, which led to water erosion of the soil (eroded forms of cambic soils). In soils affected by acidification, the value of the soil reaction decreased to 3.7, the saturation of the sorption complex dropped significantly, and the content of exchangeable Fe and Al increased. The number of plant species also decreased, and the soil without vegetation cover began to undergo intense water erosion (Madaras et al. 1999). Based on the determined amount of organic matter, however, the humus content is medium to high, which was also confirmed by analyses from 2019, and only three plots, Š1 – Š3, were investigated (Table 1).
After the building of a system of pools for capturing the flow of the AMD, Šottník and Šucha (2001) recorded a significant change in the pH, from 2.1 – 2.4 (ultra acidic) to 6.2 – 6.8 (weak acid to neutral), and an equally significant decrease in Fe, from 2260 mg/l to 39.6, and in Al, from 900 mg/l to 0.2, which Frankovská et al. (2010) later confirmed. Later studies recorded a repeat acidification of the soil environment. Čaja and Zima (2014) assessed the changes in the soil reaction from 1998 to 2014 and determined that on the area with vegetation the pH values remained at the same level as in 1998, and a positive rise in pH occurred only in the degraded area. In conclusion, they state that despite the remedial measures carried out, the soils around the heap remain extremely acidic and the threat to the environment associated with extreme acidification still persists. Čaja and Dlapa (2016a, 2016b), using infrared spectroscopy, found in degraded soils a comparable representation of original organic and mineral components as in both the reference and the degraded soils. The main change in degraded soils is the accumulation of secondary ferrous minerals, in particular ferrihydrite and jarosite. The degraded soils also showed changes in structural properties by lowering the presence of small textural pores, which increase the tendency of soils to desiccate over long dry periods. This can have a negative impact on the biological properties of degraded soils, their revival and the succession of vegetation. Extreme acidification caused by acidic sulphate waters also affects physical processes in soils, particularly the infiltration of water into the soil, which can be explained by the precipitation of secondary ferrous minerals during the interaction of acidic sulphate waters with soils (Čaja and Hrabovský 2016).
Since 2020, we have expanded monitoring at the Šobov locality to six plots, Š1 – Š6. The development, or changes in the acidity of the soil environment and its chemical indicators from 1993 to 2023 are documented in Table 1. In 2020, we even recorded the lowest overall pH value (2.75) for the whole monitoring period at the Šobov location, in a grassy meadow (Š 5). At the time of the sampling, however, it was raining heavily and the AMD, with a pH value of 3.1, was also flowing down the slope through the grass. We also recorded at that time the highest ever value of reactive Fe (32,600 mg/kg) at the mentioned location. Unlike 2022 and 2023, when we took soil samples in the same growing season when it was very warm and dry, the pH values showed a slightly acidic to neutral level and the value of reactive Fe also decreased. At other plots, the pH values continued to range from ultra-acidic to strongly acidic. Similarly, the values of organic carbon fluctuate, ranging from medium to high content. This is probably influenced by the soil sampling. The values of reactive Al and reactive Fe are high in all soil samples (Table 1).
From the above-mentioned analyses, it is evident that the soils are still contaminated, but no more polluted water is flowing into the plots. The research plots on the slope below the heap are situated in a single line in the order Š6 < Š3 < Š2 < Š1 < Š5, and their contamination increases from left to right. Š6 is the reference plot and represents the soil properties before the environmental load. Š3 is a plot showing a moderate effect of acidification, while Š2 is more acidified, though both plots have a continuous vegetation cover. Š1 represents an acidified plot, where the absence of vegetation cover in the past caused erosion. At present, the surface is only sporadically covered with vegetation. Water erosion carried away the humus horizon, revealing a subsurface skeletal horizon of subsoil weathering. The soil at plot Š5 is still affected by the AMD leaking from the heap, which flows through an erosion channel. The soil here is without vegetation cover. The humus horizon is eroded, and the subsurface horizon is marked by flowing waters, which are manifested by pseudogley processes. Plot Š4 is forested land situated below a pool built to capture acidic waters.
According to the IUSS Working Group WRB (2022), the soil type at plot Š1 is classified as Dystric Cambisol (contaminated and eroded), at plot Š2 as Dystric Cambisol (contaminated), at plot Š3 as Eutric Cambisol, at plot Š4 as Dystric Cambisol, at plot Š5 as Stagnic Dystric Cambisol (contaminated and eroded), and at plot Š6 as Eutric Cambisol.
Plant community structure
The vegetation on the plots can be defined as follows:
-
Plot Š1: The vegetation is poorly developed and is composed of scattered individuals of acidophilous grasses (Agrostis stolonifera, Avenella flexuosa) and herbs (Acetosella vulgaris).
-
Plot Š2: Open species-poor acidophilous grassland. Floristic composition:
-
E1: 60%, E0: 5%
-
E1: Agrostis stolonifera 3, Avenella flexuosa 3, Acetosella vulgaris 2, Festuca rubra agg. 1, Pilosella officinarum 1, Carex hirta + , Nardus stricta +
-
-
Plot Š3: Dense, relatively species-rich grassland formed by common mesophilous meadow species.
-
E1: 100%
-
E1: Arrhenatherum elatius 3, Festuca rubra agg. 3, Leontodon hispidus 2, Dactylis glomerata 1, Trisetum flavescens 1, Alopecurus pratensis 1, Anthoxnathum odoratum 1, Jacea phrygia 1, Poa pratensis 1, Trifolium repens 1, Acetosa pratensis + , Achillea millefolium agg. + , Leucanthemum vulgare + , Lotus corniculatus + , Pimpinella saxifraga + , Plantago lanceolata + , Ranunculus acris + , Stellaria graminea + , Tragopogon orientalis + , Trifolium pratense + , Veronica chamaedrys + , Vicia hirsuta + , Vicia sepium + , Rumex sp. r
-
-
Plot Š4: without vegetation cover.
-
Plot Š5: The vegetation is poorly developed and is composed of scattered individuals of acidophilous grasses (Agrostis stolonifera, Avenella flexuosa) and herbs (Acetosella vulgaris).
-
Plot Š6: Dense, species-rich mesophilous semi-natural grassland. Floristic composition:
-
E1: 100%
-
E1: Arrhenatherum elatius 4, Cerastium holosteoides 2, Convolvulus arvense 2, Poa pratensis 2, Trisetum flavescens 2, Dactylis glomerata 1, Myosotis arvensis 1, Acetosa pratensis + , Achillea millefolium agg. + , Anthoxanthum odoratum + , Crepis biennis + , Elytrigia repens + , Jacea phrygia + , Knautia arvensis + , Leucanthemum vulgare + , Lotus corniculatus + , Lychnis flos-cuculi + , Medicago lupulina + , Plantago lanceolata + , Ranunculus acris + , Tragopogon orientalis + , Trifolium pratense + , Veronica chamaedrys + , Vicia sepium +
-
The presented physico-chemical properties of the soils at the Šobov locality, their changes and the plant community have a significant effect on the biological and microbiological properties of the soils, including the diversity of soil mycocenosis (Balogová 2022; Nosalj et al. 2021).
Mycocenosis of the Šobov locality
The occurrence of microscopic filamentous fungi at plots Š1 – Š3 at the Šobov locality, where research has been conducted since 1993, has been documented by Adamcova (2010), Holub et al. (1993), Nosalj et al. (2021), Nováková et al. (2012), Šimonovičová (2013), Šimonovičová et al. (2019) and Výbohová et al. (1999). Over the entire monitored period, 36 genera and 91 species of microscopic filamentous fungi have been isolated (Table 2). Zygomycota (7 genera and 10 species) make up 11.0% of the total; Ascomycota (28 genera and 80 species) make up 87.9% of the total, and Basidiomycota 1.1% (1 genus and 1 species).
Zygomycota are represented in the individual soil samples by almost the same number of representatives, and species of the genera Absidia and Zygorhynchus were present in each soil sample. The species Absidia glauca and Mucor racemosus f. racemosus occurred only in the soil sample from plot Š1 and the species Absidia cylindrospora var. cylindrospora and Zygorhynchus heterogamus occurred only in the soil sample from plot Š3. The diversity of this group of microscopic filamentous fungi is relatively low, however.
On the other hand, the diversity of microscopic filamentous fungi from the systematic group Ascomycota is notably high. Species of the genus Penicillium are the most represented (35); however, among them only the species Penicillium chrysogenum var. chrysogenum and Penicillium fuscum (isolated as Eupenicillium pinetorum) were found in soil samples from all three plots Š1 – Š3. The species Penicillium daleae, Penicillium italicum, Penicillium restrictum and Penicillium sacculum (isolated as Eladia saccula) were found only in the soil sample from plot Š1. The species Penicillium adametzii, Penicillium freii, Penicillium glabrum, Penicillium islandicum, Penicillium ochrochloron, Penicillium spinulosum and Penicillium steckii were isolated only in the soil sample from plot Š2. The species Penicillium glandicola var. glandicola, Penicillium javanicum (isolated as Eupenicillium javanicum), Penicillium crustaceum (isolated as Eupenicillium crustaceum) and Penicillium vulpinum were found only in the soil sample from plot Š3. The second most abundant genus is Aspergillus (7 species), with the species Aspergillus fischeri (isolated as Neosartorya fischeri), which was found in soil samples from all three plots Š1 – Š3. The genus Trichoderma is represented by 5 species, and the species Trichoderma koningii, Trichoderma viride and Trichoderma sp. are represented in the soil sample from plots Š1 – Š3. The genus Cladosporium is represented by 4 species, with the most abundant being Cladosporium cladosporioides, which was present in soil samples from all three plots Š1 – Š3. Other genera are represented by a smaller number of species, such as Hamigera (3 species) or Paecilomyces (3 species). From the overall representation of Ascomycota, the species Aspergillus strictus (isolated as Emericella striata), Aureobasidium sp., Chaetomium globosum, Cladosporium pseudocladosporioides, Epicoccum nigrum, Hamigera insecticola, Isaria sp., Trichoderma harzianum and Verticillium sp. occurred only in the soil samples from plot Š1. The species Acremonium sp., Aspergillus oryzae, Emericella quadrilineata, Fusarium chlamydosporum var. chlamydosporum, Hamigera avellanea, Paecilomyces niveus (isolated as Byssochlamys nivea), Staphylotrichum sp. and Tolypocladium cylindrosporum occurred only in the soil samples from plot Š2. The species Aspergillus awamori, Aspergillus versicolor, Aspergillus wentii, Bionectria sesquicillii, Botryotrichum piluliferum, Phialophora fastigiata, Staphylotrichum coccosporum, Trichoderma hamatum and Trichophaea abundans were isolated only in the soil sample at plot Š3. Basidiomycota are represented only by the species Bjerkandera adustata in the soil sample at plot Š2. The diversity of microscopic filamentous fungi that were isolated and identified during the years 1993–2021 is lowest at plot Š1 (41 species). Plot Š2 (58 species) dominates significantly, followed by plot Š3 (48 species) (Table 2). Adamcova (2010) also analysed soil samples within a 24 m long transect in the direction of plot Š6. From the isolated species of microscopic filamentous fungi that did not occur at plots Š1 – Š3 (Table 2), the author mentions Absidia glauca var. glauca, Aspergillus flavus, Beauveria bassiana, Humicola sp., Penicillium griseofulvum, Penicillium olsonii and Penicillium pullvilorum.
The genera and species of the identified microscopic filamentous fungi belong among the ubiquitous representatives of the soil mycobiome. They also occur very often in soils and solid substrates contaminated with several heavy metals and potentially toxic elements (Pathak et al. 2020; Redkina et al. 2020; Šimonovičová et al. 2019; Jeszeová et al. 2018; Zotti et al. 2014), and the most common are genera of the species Aspergillus, Memnoniella, Penicillium, Clonostachys and Trichoderma. According to Hujslová et al. (2017), an ultra-acidic soil environment might potentially offer new biotechnologically interesting fungi, especially in terms of enzyme production. Also, thermophilic fungi, particularly the species Talaromyces but also Aspergillus, Cladosporium and Trichoderma, can represent a rich source of industrially relevant enzymes at pH 2.0 (Gao et al. 2021; Thang et al. 2019). The effect of soil acidification showed that pH is an essential predictor for controlling the distribution of microbial communities, and fungal communities exhibit little response to soil acidity (Wang et al. 2022).
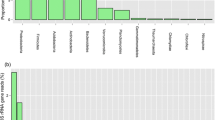
Within the expanded research from three to six study plots, we focused on keratinophilic species of microscopic filamentous fungi (Fig. 6), and a total of 39 keratinophilic microscopic fungi were isolated from the soil samples (Table 3 and 4). We recorded the occurrence of 16 keratinophilic species, one of which, i.e. Trichophyton ajelloi (representative of the order Onygenales), was keratinolytic (Fig. 7). Species of the genus Penicillium were isolated as keratinophiles from the three plots in the previous study (Adamcová et al. 2012) (Table 4). The species Penicillium brasillianum and P. minioluteum occurred only in the soil sample from plot Š3, and P. herquei and P. janthinellum only in the soil sample from plot Š1. Purpureocillium lilacinum (6a) and Keithomyces carneus (6b) were the most frequent representatives at all six study plots. The species Metapochonia bulbillosa (6c) occurred in soil samples from all the plots, with the exception of Š1 and Š6. The species Pochonia chlamydosporia and Metarhizium anisopliae occurred only in the soil sample from plot Š2, Flavocillium bifurcatum (6d) in the soil samples from plots Š1 and Š5, Gliomastix murorum (6e) in the soil sample from plot Š2 and together with Clonostachys rosea (6f) in the soil sample from plot Š3. The species Lecanicillium psalliotae and species of the genus Tritirachium occurred only in soil samples from plot Š4. A new species from the genus Metapochonia (manuscript in preparation) was also isolated; it was recorded only in the soil sample from plot Š2.
The occurrence of keratinophilic species of fungi is influenced by several ecological factors, such as temperature, humidity, soil reaction, amount of carbon, nitrogen and sulphur, potentially heavy metals and others (Garg et al. 1985). Several authors (Bohacz et al. 2022; Sharma and Swati 2014), however, consider the effect of soil reaction to be the most important factor. Most keratinophilic fungal species prefer a neutral to weakly alkaline soil reaction with a high content of organic substances (Böhme and Ziegler 1969; Korniłłowicz-Kowalska and Bohacz 2002; Sharma and Swati 2014); some of them, however, also tolerate a more acidic environment. Keratinophilic fungal species have been found to occur in soils with pH values ranging from 3.4 – 9.0 (Bohacz et al. 2022; Javoreková et al. 2012; Kačinová et al. 2013). The kertinophilous species from our study occurred mainly in samples with extremely acidic to strongly acidic soil reaction (pH 3.7 – 5.5). The greatest diversity (8 species) was recorded precisely at plot Š2, the plot with an extremely acidic soil reaction (pH 3.7), which is covered with acidophilic vegetation, similar to the previous study when determining the total mycocenosis (Nosalj et al. 2021). Some keratinophilic species of fungi are able to survive under extreme conditions, such as in this case, in acidification and the occurrence of potentially toxic elements. Their ability to adapt and produce a broad range of metabolites represents a great potential for use in many industries (Danko et al. 2021; Kumar et al. 2021).
Among the keratinolytic fungi, we recorded only the species Trichophyton ajelloi (sexual morph Arthroderma uncinatum) (7 a-e), which was isolated from soil samples with an extremely acidic soil reaction (pH 3.7) at plot Š2 and at the reference plot (Š6) with a neutral soil reaction (pH 6.8). Several studies have confirmed its occurrence in environments with an acidic soil reaction (Bohacz et al. 2022; Javoreková et al. 2012). Adamcová et al. (2012) recorded the occurrence of Trichophyton ajelloi at the Šobov locality, particularly in samples that were least affected by acidification, and even though this species is considered acidophilic and acid-tolerant, it occurs quite often in the soil across the entire pH range (Garg et al. 1985; Hubálek 2000; Javoreková et al. 2012). The lower representation of Trichophyton ajelloi in the soil samples from Šobov may be due to the strong acidification and contamination of the environment with potentially toxic elements.
Keratinolytic species of fungi play a significant role in the decomposition of keratin-containing substrates; thus, they are naturally involved in the cycle of elements in the ecosystem (Bohacz et al. 2022). The relatively low representation of keratinolytic fungal species may indicate the inhibition of decomposition processes in acidified soils, such as the land at the Šobov locality.
Keratinophilic fungi survive in various extreme environmental conditions and are known to produce a number of bioactive metabolites. The determination of keratinophilic fungi is of great importance because of their specific nature as they can be novel sources of many promising metabolites for use in medicine, biotechnology and agrochemistry (Kumar et al. 2021).
Conclusion
More than 20 years after remedial measures were carried out which were supposed to mitigate the impact of acidification after the extraction of quartz rock, we specified in more detail the six studied plots (Š1 – Š6) at the Šobov locality. The plots differ from one another in physico-chemical properties and plant communities. The soil type at plot Š1 is Dystric Cambisol (contaminated and eroded), at plot Š2 Dystric Cambisol (contaminated), at plots Š3 and Š6 Eutric Cambisol, at plot Š4 Dystric Cambisol and at plot Š5 Stagnic Dystric Cambisol (contaminated and eroded). From the pedological point of view, the physico-chemical differences between the soils at these plots are not the result of pedogenesis, but rather the effect of extremely acidic mineralised solutions that have flowed or are still flowing from the heap. The plant community is most often poor to weakly developed with acidophilic vegetation (Š1, Š2, Š5), or without vegetation (Š4) or with dense species-rich vegetation (Š3, Š6). The study of saprotrophic microscopic filamentous fungi, which took place from 1993 to 2021 at plots Š1 – Š3, confirmed a significant suppression of representatives from the phylum Zygomycota and their relatively low diversity. Species of the genera Absidia and Zygorhynchus occurred the most frequently in the soil samples. In contrast, the diversity of the phylum Ascomycota is quite high. The genus Penicillium has a dominant representation (35 species), and only the species Penicillium chrysogenum var. chrysogenum occurred repeatedly in the samples. We isolated as many as 10 heat-resistant species, e.g. species of the genus Eupenicillium and Talaromyces. The genus Aspergillus is represented by 7 species, with Aspergillus fisheri and Aspergillus niger among the most frequently isolated. The species Trichoderma koningii and Trichoderma viride were also among the most frequently isolated. We supplemented the mycobiome of the Šobov locality with the analysis of keratinophilic species of microscopic fungi. Of the 15 species of keratinophilic fungi, Purpureocillium lilacinum and Keithomyces carneus were the most common. We recorded keratinolytic properties (the ability to break down keratin) only in the species Trichophyton ajelloi.
On the basis of physico-chemical analyses, we can clearly confirm that acidification of the territory in the Šobov locality continues, since soil samples from plots Š1 – Š5 are ultra-acidic to strongly acidic. Soil reaction is therefore the most important ecological factor that influences the biological properties of soils.
Data Availability
The data presented in this study are available on request from the corresponding author.
References
Adamcova D (2010) Biodiversity of filamentous microscopic fungi isolated from soils influenced by acid mine drainage in Šobov region (Banska Štiavnica, Slovakia). Phytopedon 9:1–5 ISSN 1336-1120
Adamcová D, Labuda R, Šimonovičová A (2012) Keratinophilic fingi of Dystric Cambisoil in Šobov locality near Banská Štiavnica. Phytopedon (Bratislava) 11:10–13. ISSN 1336-1120
Balogová M (2022) Mikrobiálna aktivita kambizeme na acidifikovanom území bývalého ťažobného areálu Šobov. Phytopedon (Bratislava) 21:6–10. ISSN 1336-1120
Bohacz J, Możejko M, Korniłłowicz-Kowalska T, Siebielec G (2022) Impact of ecological factors on the occurrence and spatial-taxonomic structure of keratinophilic fungi and their co-occurrence in arable soils. Agriculture 12:194. https://doi.org/10.3390/agriculture12020194
Böhme H, Ziegler H (1969) The distribution of geophilic dermatophytes and other keratinophilic fungi in relation to the pH of the soil. Mycopathol Mycol Appl 38:247–255. https://doi.org/10.1007/BF02052677
Braun-Blanquet J (1964) Pflanzensoziologie, grundzüge der vegetationskunde, 3rd edn. Springer-Verlag, Berlin, p 631
Čaja A, Dlapa P (2016a) Investigation of degraded soils at Šobov location (Banská Štiavnica) by infrared spectroscopy (in Slovak). Phytopedon (Bratislava) 15:41–44. ISSN 1336-1120
Čaja A, Dlapa P (2016b) Changes of water retention properties in soils affected by extreme acidification (in Slovak). 15:51–55. ISSN 1336–1120
Čaja A, Hrabovský A (2016) Influence of extreme acidification on water infiltration into soil (in Slovak). Phytopedon (Bratislava) 15:41–46. ISSN 1336-1120)
Čaja A, Zima L (2014) Evaluation of changes in soil pH at Šobov (Banská Štiavnica) since 1998 until present (in Slovak). Phytopedon (Bratislava) 13:65–72. ISSN 1336-1120
Danko M, Mosnáčková K, Vykydalová A, Kleinová A, Puškárová A, Pangallo D, Bujdoš M, Mosnáček J (2021) Properties and degradation performances of biodegradable poly (lactic acid)/poly (3-hydroxybutyrate) blends and keratin composites. Polymers 13:2693
de Hoog GS, Guarro JG, Gené J, Ahmed S, Al-Hatmi AMS, Figueras MJ, Vitale RG (2020) Atlas of clinical fungi, 4th edn. Foundation Atlas of Clinical Fungi, Hilversum, Utrecht
Frankovská J, Kordík J, Slaninka I, Jurkovič Ľ, Greif V, Šottník P, Dananaj I, Mikita S, Dercová K, Jánová V (2010) Atlas of rehabilitation methods of environmental burdens (in Slovak). Štátny geologický ústav Dionýza Štúra, Bratislava 360 p. ISBN 978-80-89343-39-3
Gao H, Wang Y, Luo Q, Yang L, He X, Wu J, Kachanuban K, Wilaipun P, Zhu W, Wang Y (2021) Bioactive metabolites from acid-tolerant fungi in a Thai mangrove sediment. Front Microbiol 11:609952. https://doi.org/10.3389/fmicb.2020.609952
Garg AP, Gandotra S, Mukrji KG, Pugh GJF (1985) Ecology of keratinophilic fungi. Proc Plant Sci 94:149–163
Holub Z, Šimonovičová A, Banásová V (1993) The influence of acidification on some chemical and microbiological properties of soil, those determining plant viability. Biológia, Bratislava 48(6):671–675
Hrivňáková K, Makovníková J, Barančíková G, Bezák P, Bezáková Z, Dodok R, Grečo V, Chĺpik J, Kobza J, Lištijak M, Mališ J, Píš V, Schlosserová J, Slávik O, Styk J, Siráň M (2011) Unified workflow analyzes of soils (Output of the research project „Monitoring and evaluation of the properties of soil in Slovakia and potential for their development“). Research Institute for Soil Science and Conservation, Bratislava, p 136
Hubálek Z (2000) Keratinophilic fungi associated with free living mammals and birds. In: Kushwaha RKS, Guarro J (eds) Biology of dermatophytes and other keratinophilic fungi. Revista Iberoamericana de Micología, Bilbao 17, pp 104–108
Hujslová M, Kubátová A, Bukovská P, Chudíčková M, Kolařík M (2017) Extremly acidic soils are dominated by species-poor and highly specific fungal communities. Microbiol Ecol 73:321–337. https://doi.org/10.1007/s00248-016-0860-3
IUSS Working Group WRB (2022) World Reference Base for Soil Resources. International soil classification system for naming soils and creating legends for soil maps, 4 edn. International Union of Soil Sciences (IUSS), Vienna
Javoreková A, Labuda R, Maková J, Novak J (2012) Keratinophilic fungi isolated from soils of long-term fold-grazed, degraded pastures in National Parks of Slovakia. Mycopathologia 174:239–245
Jeszeová L, Puškárová A, Bučková M, Kraková L, Grivalský T, Danko M, Mosnáčková K, Chmela Š, Pangallo D (2018) Microbial communities responsible for the degradation of poly (lactic acid)/poly (3-hydroxybutyrate) blend mulches in soil burial respirometric tests. World J Microbiol Biotechnol 34:1–12
Korniłłowicz-Kowalska T, Bohacz J (2002) Some correlations between the occurrence frequency of keratinophilic fungi and selected soil properties. Acta Mycol 37:101–116
Kačinová J, Tančinová D, Labuda R (2013) Keratinophilic fungi in soils stressed by occurrence of animals. J Microbiol Biotechnol Food Sci 2:1436–1446
Kumar J, Singh I, Kushwaha R (2021) Keratinophilic fungi: diversity. Environmental and Biotechnological Implications. https://doi.org/10.1007/978-981-16-2350-9_15
Labuda R, Bernreite A, Hochenauer D, Al K, Kandemir H, Schüller C (2021) Molecular systematics of Keratinophyton: the inclusion of species formerly referred to Chrysosporium and description of four new species. IMA Fungus 12:17. https://doi.org/10.1186/s43008-021-00070-2
Madaras M, Dlapa P, Banásová V (1999) Zonality of soil nad vegetational degradation at locality Banská Štiavnica-Šobov. Proceedings No, 22, pp 109–116
Marhold K, Hindák F (1998) (eds) Checklist of non-vascular and vascular plants of Slovakia. Veda, Bratislava, pp 687
Nosalj S, Šimonovičová A, Pauditšová E, Hanajík P, Vojtková H, Benková B (2021) Diversity of soil microscopic filamentous fungi in Dystric Cambisol at the Banská Štiavnica – Šobov (Slovakia) locality after application of remediation measures. Biologia 76:2123–2131
Nováková A, Šimonovičová A, Kubátová A (2012) List of cultivable microfungi from soils, soil related substrates and underground environment of the Czech and Slovak republics. Mycotaxon 119:593
Pathak A, Jaswal R, Xu X, White JR, Edwards B III, Hunt J, Brooks S, Rathore RS, Agarwal M, Chauhan A (2020) Characterization of bacterial and fungal assemblages from historically contaminated metalliferous soils using metagenomics coupled with diffusion chambres and microbial traps. Front Microbiol 11:1024. https://doi.org/10.3389/fmicb.2020.01024
Redkina VV, Shalygina RR, Korneykova MV (2020) Microfungi, algae and cyanobacteria in soils polluted with fluorine (Kola Peninsula, Russia). Czek Polar Research 10:1. https://doi.org/10.5817/CPR2020-1-9
Sharma R, Swati K (2014) Prevalence of keratinophilic fungi at various pH in different areas of Jaipur, Rajasthan. J Microbiol Biotechnol 4:17–21
Šimonovičová A (2013) Biodiversity of microscopic fungi in soil types of Slovakia (in Slovak). Univerzita Komenského, Bratislava, p 82
Šimonovičová A, Kraková L, Pauditšová E, Pangallo D (2019) Occurrence and diversity of cultivable autochthonous microscopic fungi in substrates of old environmental loads from mining acitivites in Slovakia. Ecotoxicol Environ Safe 172:194–202. https://doi.org/10.1016/j.ecoenv.2019.01.064
Šottník P, Šucha V (2001) Remediation of acid mine drainage from Šobov mine-banská Štiavnica (in Slovak). Mineralia Slovaca 33:53–60
Thang VN, Thuy NT, Huong HTT, Hiem DD, Hang DTM, Anh DTK, Hűttner S, Larsbrink J (2019) Surveying of acid-tolerant thermophilic lignocellulolytic fungi in Vietnam reveals surprisingly high genetic diversity. Sci Rep 9:3674. https://doi.org/10.1038/s41598-019-40213-5
Výbohová M, Šimonovičová A, Dlapa P, Madaras M (1999) Microbial activity in soils under the influence of pyrite weathering. Geologica Carpatica 50:389–394
Wang T, Cao X, Chen M, Lou Y, Wang H, Yang Q, Pan H (2022) Effects of soil acidification on bacterial and fungal communities in the Jiaodong peninsula, Northern China. Agronomy 12:927. https://doi.org/10.3390/agronomy12040927
Zotti M, Di Piazza S, Roccotiello E, Lucchetti G, Mariotti MG, Marescotti P (2014) Microfungi in highly copper-contaminated soils from an abandoned Fe-Cu sulphide mine: growth responses, tolerance and bioaccumulation. Chemosphere 117:471–476
Acknowledgements
The work was supported by Slovak National Grant Agency – project VEGA 1/0194/21.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Contributions
Conceptualization, original draft preparation and methodology, AŠ; Writing—review and editing, mycological analyses and visualization, SN; manuscript review and editing, soil types classification, AD; manuscript review and editing, methodology and identification of keratinophilic fungi, RL; plant community structure determination, manuscript review and editing, JK. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nosalj, S., Hrabovský, A., Labuda, R. et al. Biological and physico-chemical properties of the soil in an acidified environment influenced by previous mining activities at the Šobov locality (SLOVAKIA). Biologia (2024). https://doi.org/10.1007/s11756-024-01638-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11756-024-01638-0