Abstract
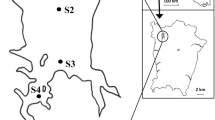
The influence of physicochemical parameters other than the nutrients load on the community structure of phyto- and zooplankton in lowland shallow lakes is still poorly understood. In this study we investigated the structure of the plankton community in Danube Delta (Romania) and the relationships with environmental variables. Among the 206 taxa observed, 33 species were dominant. Canonical Correspondence Analysis indicated that incident light, lakes depth, surface area and water conductivity were of significant importance in controlling the variation in the structure of the plankton assemblages. The resulted models from averaging regression and cross-calibration predicted the main environmental parameters and allowed the selection of phyto and zooplankton species as potential biological indicators. Weighted averaging regression and cross-calibration generated useful models for predicting the main four investigated environmental parameters, which contribute to the selection of phyto- and zooplankton species as potential biological indicators.




Similar content being viewed by others
References
Armengol X, Esparcia A, Miracle M (1998) Rotifer vertical distribution in a strongly stratified lake: a multivariate analysis. Hydrobiologia 387:161–170. https://doi.org/10.1023/A:1017054129742
Armengol X, Miracle XR (1999) Zooplankton communities in doline lakes and pools, in relation to some bathymetric parameters and physical and chemical variables. J Plankton Res 21:2245–2261. https://doi.org/10.1093/plankt/21.12.2245
Baloch WA, Suzuki H, Onoue Y (2000) Occurrence of planktonic rotifer, Filinia longiseta in southern Kyushu, Japan. Pak J Zool 32:279–281
Basińska A, Kuczyńska-Kippen N, Świdnicki K (2010) The body size distribution of Filinia longiseta(Ehrenberg) in different types of small water bodies in the Wielkoposka region. Limnetica 29:171–182. https://doi.org/10.23818/limn.29.14
Baumert AJ (1998) The components of feeding behavior in the rotifer Asplanchna herricki: attack, capture, consumption, selectivity, and trophi morphology. Honors Theses, St. John's University
Bērziņš B, Pejler B (1989a) Rotifer occurrence and trophic degree. Hydrobiologia 182:171–180. https://doi.org/10.1007/BF00006043
Bērziņš B, Pejler B (1989b) Rotifer occurrence in relation to temperature. Hydrobiologia 175:223–231. https://doi.org/10.1007/BF00006092
Birks HJB, Line JM, Juggins S, Stevenson AC, Ter Braak CJF (1990) Diatoms and pH reconstruction. Phil. Trans. R. Soc. Lond. Ser. B 327:263–278. https://doi.org/10.1098/rstb.1990.0062
Burdíková Z, Čapek M, Švindrych Z, Gryndler M, Kubínová L, Holcová K (2012) Ecology of testate amoebae in the Komořany ponds in the Vltava Basin. Microb Ecol 64:117–130. https://doi.org/10.1007/s00248-011-0003-9
Catherine A, Escoffier N, Belhocine A (2012) On the use of the FluoroProbe(r), a phytoplankton quantification method based on fluorescence excitation spectra for large-scale surveys of lakes and reservoirs. Water Res 46:1771–1784. https://doi.org/10.1016/j.watres.2011.12.056
Chang K-H, Doi H, Nishibe Y, Nakano S- Ichi (2010) Feeding habits of omnivorous Asplanchna: comparison of diet composition among Asplanchna herricki, A priodonta and A girodi in pond ecosystems J Limnol 692:209–216. https://doi.org/10.4081/jlimnol.2010.209
Christie CE, Smol JP (1993) Diatom assemblages as indicators of lake trophic status in southeastern Ontario lakes. J Phycol 29:575–586. https://doi.org/10.1111/j.0022-3646.1993.00575.x
Clement P, Wurdak E, Amsellem J (1983) Behavior and ultrastructure of sensory organs in rotifers. Hydrobiologia 104:89–129. https://doi.org/10.1007/BF00045957
Coops H, Buijse LL, Buijse AD, Constantinescu A, Covaliov S, Hanganu J, Ibelings BW, Menting G, Navodaru I, Oosterberg W, Staras M, Török L (2008) Trophic gradients in a large-river Delta: ecological structure determined by connectivity gradients in the Danube Delta (Romania). River Res Appl 24:698–709. https://doi.org/10.1002/rra.1136
Coops H, Hanganu J, Tudor M, Oosterberg W (1999) Classification of Danube Delta lakes based on aquatic vegetation and turbidity. In: Caffrey J, Barrett PRF, Ferreira MT, Moreira IS, Murphy KJ, Wade PM (eds) Biology, Ecology and Management of Aquatic Plants. Developments in Hydrobiology, vol 147. Springer, Dordrecht, pp187–191. https://doi.org/10.1007/978-94-017-0922-4_26
Cornillac A, Wurdak E, Clément P (1983) Phototaxis in monochromatic light and microspectrophotometry of the cerebral eye of the rotifer Brachionus calyciflorus. Hydrobiologia 104:191–196. https://doi.org/10.1007/BF00045967
Damian-Georgescu A (1963) Fauna Republicii Socialiste Romania. Crustacea Vol.IV, Fascicola 6, Copepoda.Cyclopidae. Editura Academiei Române, București
Damian-Georgescu A (1966) Fauna Republicii Socialiste Romania. Crustacea Vol.IV, Fascicula 8, Copepoda. Calanoida. Editura Academiei Române, București
Damian-Georgescu A (1970) Fauna Republicii Socialiste Romania. Crustacea Vol.IV, Fascicula 11, Copepoda.Harpacticoida. Editura Academiei Române, București
de Carvalho LR, Pipole F, Werner VR, Laughinghouse Iv HD, de Camargo AC, Rangel M, Konno K, Sant' Anna CL (2008) A toxic cyanobacterial bloom in an urban coastal lake, Rio Grande do Sul state, Southern Brazil. Braz J Microbiol 39:761–769. https://doi.org/10.1590/S1517-838220080004000031
Deng J, Qin B, Paerl HW, Zhang Y, Wu P, Ma J, Chen Y (2014) Effects of nutrients, temperature and their interactions on spring phytoplankton community succession in Lake Taihu, China. PLoS One 9: e113960. https://doi.org/10.1371/journal.pone.0113960
Dodson SI, Arnott SE, Cottingham KL (2000) The relationship in lake communities between primary productivity and species richness. Ecology 81:2662–2679. https://doi.org/10.1890/0012-9658(2000)081[2662:TRILCB]2.0.CO;2
Domingues RB, Guerra CC, Barbosa AB, Galvão HM (2015) Are nutrients and light limiting summer phytoplankton in a temperate coastal lagoon? Aquat Ecol 49:127–146. https://doi.org/10.1007/s10452-015-9512-9
Duggan IC, Green JD, Shiel RJ (2002) Distribution of rotifer assemblages in North Island, New Zealand, lakes: relationships to environmental and historical factors. Freshw Biol 47:195–206. https://doi.org/10.1046/j.1365-2427.2002.00742.x
Dumont HJ (1977) Biogeography of rotifers. Hydrobiologia 104:19–30. https://doi.org/10.1007/BF00045948
Edmonson WT, Winberg GG (1971) A manual on the methods for the assessment of secondary productivity in freshwaters (IPB handbook 17). Blackwell Scientific Publications, Oxford and Edinburg
Ettl H (1978) Xanthophyceae, Teil 1, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Jena, Stuttgart-New York
Ettl H (1983a) Chlorophyta I, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Jena, Stuttgart-New York
Ettl H (1983b) Chlorophyta I, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Jena, Stuttgart-New York
Foissner W, Blatterer H, Berger H, Kohmann F (1991) Taxonomical and ecological revision of Ciliata from saprobic systems, volume I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea, reports by the Bavarian state Office for Water Management, 1 (91). Informationsberichte des Bayer Landesamtes für Wasserwirtschaft, Munich
Foissner W, Blatterer H, Berger H, Kohmann F (1992) Taxonomical and ecological revicion of Ciliata from saprobic systems, volume II: Peritrichia, Heterotrichida, Odontostomatida. Reports by the Bavarian state Office for Water Management, 5 (92). Informationsberichte des Bayer Landesamtes für Wasserwirtschaft, Munich
Foissner W, Berger H, Kohmann F (1994) Taxonomical and ecological revision of Ciliata from saprobic systems, volume III: Hymenostomata, Prostomatida, Nassulida. Reports by the Bavarian state Office for Water Management, 1 (94). Informationsberichte des Bayer Landesamtes für Wasserwirtschaft, Munich
Foissner W, Berger H, Kohmann F (1995) Taxonomical and ecological revision of Ciliata from saprobic systems, volume IV: Gymnostomatea, Loxodes, Suctoria. Reports by the Bavarian state Office for Water Management, 1 (95). Informationsberichte des Bayer Landesamtes für Wasserwirtschaft, Munich
Gâştescu P (2009) The Danube Delta biosphere reserve. Geography, biodiversity, protection, management. Rev Roum Géogr 53:139–152
Gilbert JJ, Hampton SE (2001) Diel vertical migrations of zooplankton in a shallow, fishless pond: a possible avoidance- response cascade by notonectids. Freshw Biol 46:611–621. https://doi.org/10.1046/j.1365-2427.2001.00697.x
Grospietsch T (1972)Wechsel-tierchen (Rhizopoden). Kosmos-Verlag Franckh, Stuttgart
Gulati RD (1990) Zooplankton structure in the Loosdrecht lakes in relation to trophic status and recent restoration measures. Hydrobiologia 191:173–188. https://doi.org/10.1007/BF00026051
Houliez E, Lizon F, Thyssen M, Artigas LF, Schmitt FG (2012) Spectral fluorometric characterization of haptophyte dynamics using the FluoroProbe: an application in the eastern English Channel for monitoring Phaeocystis globosa. J Plankton Res 34:136–151. https://doi.org/10.1093/plankt/fbr091
Interlandi SJ, Kilham SS (2001) Limiting resources and the regulation of diversity in phytoplankton communities. Ecology 82:1270–1282. https://doi.org/10.1890/0012-9658(2001)082[1270:LRATRO]2.0.CO;2
Jeppesen E, Jensen JP, Søndergaard M, Lauridsen T, Landkildehus F (2000) Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshw Biol 45:201–218. https://doi.org/10.1046/j.1365-2427.2000.00675.x
Kasprzak P, Padisák J, Koschel R, Krienitz L, Gervais F (2008) Chlorophyll a concentration across a trophic gradient of lakes: an estimator of phytoplankton biomass? Limnologica 38:327–338. https://doi.org/10.1016/j.limno.2008.07.002
Kilroy C, Biggs BJF, Vyverman W, Broady PA (2006) Benthic diatom communities in subalpine pools in New Zealand: relationships to environmental variables. Hydrobiologia 561:95–110. https://doi.org/10.1007/s10750-005-1607-1
Komárek J, Anagnostidis K (1998) Cyanoprokaryota, Teil 1, Chroococcales. Süßwasserflora von Mitteleuropa, Springer Spektrum
Komárek J, Anagnostidis K (2005) Cyanoprokaryota Teil. 2, Oscillatoriales, Süßwasserflora von Mitteleuropa. Springer Spektrum
Kowaleczko M (2005) Preliminary investigations on the dependence between the day-time and vertical migrations of rotifers in Piaseczno during summer stagnation. Ann Univ Mariae Curie-Skłodowska C Biol 60:55-61
Krammer K, Lange-Bertalot H (1986) Naviculaceae I, Süßwasserflora von Mitteleuropa. Springer Spektrum
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae, Teil 2, Süßwasserflora von Mitteleuropa. Springer Spektrum
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae, Teil 3, Süßwasserflora von Mitteleuropa. Springer Spektrum
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae, Teil 4, Süßwasserflora von Mitteleuropa. Springer Spektrum
Kuznetsova EV, Kosolapov DB, Kosolapova NG, Sakharovaa EG, Krylova AV (2019) Dynamics and relationship between plankton organisms in the littoral zone of a large plain reservoir at the beginning of the vegetation season. Contemp Probl Ecol 12:575–583. https://doi.org/10.1134/S1995425519060076
Lehman CL, Tilman D (2000) Biodiversity, stability, and productivity in competitive communities. Am Nat 156:534–552. https://doi.org/10.1086/303402
Leland HV (1995) Distribution of phytobenthos in the Yakima River basin, Washington, in relation to geology, land use, and other environmental factors. Can J Fish Aquat Sci 52:1108–1129. https://doi.org/10.1139/f95-108
Leland HV, Brown LR, Mueller DK (2001) Distribution of algae in the San Joaquin River, California, in relation to nutrient supply, salinity and other environmental factors. Freshw Biol 46:1139–1167. https://doi.org/10.1046/j.1365-2427.2001.00740.x
Leland HV (2003) The influence of water depth and flow regime on phytoplankton biomass and community structure in a shallow, lowland river. Hydrobiologia 506:247–255. https://doi.org/10.1023/B:HYDR.0000008596.00382.56
Matveeva LK (1986) Pelagic rotifers of Lake Glubokoe from 1897 to 1984. Hydrobiology 141:45–54. https://doi.org/10.1007/BF00007479
Miracle MR, Armengol-Díaz J, Dasí MJ (1993) Extreme meromixis determines strong differential planktonic vertical distributions. SIL proceedings, 1922-2010. Verh Int Verein Limnol 25:705–710. https://doi.org/10.1080/03680770.1992.11900230
Munn MD, Black RW, Gruber SJ (2002) Response of benthic algae to environmental gradients in an agriculturally dominated landscape. J North Am Benthol Soc 21:221–237. https://doi.org/10.2307/1468411
Muylaert K, Pérez-Martínez C, Sánchez-Castillo P, Torben LL, Vanderstukken M, Declerck SAJ, Van der Gucht K, Conde-Porcuna JM, Jeppesen E, De Meester L, Vyverman W (2010) Influence of nutrients, submerged macrophytes and zooplankton grazing on phytoplankton biomass and diversity along a latitudinal gradient in Europe. Hydrobiologia 653:79–90. https://doi.org/10.1007/s10750-010-0345-1
Negrea Ş (1983) Fauna Republicii Socialiste Romania. Cladocera, Editura Academiei Române, București
Ochocka A, Pasztaleniec A (2016) Sensitivity of plankton indices to lake trophic conditions. Environ Monit Assess 188:622. https://doi.org/10.1007/s10661-016-5634-3
Păceșilă I (2015) Benthic microbial biomass dynamics in aquatic ecosystems of the Danube Delta. Rom Biotechnol Lett 20:10946–10503
Paxinos R, Mitchell JG (2000) A rapid Utermöhl method for estimating algal numbers. J Plankton Res 22:2255–2262. https://doi.org/10.1093/plankt/22.12.2255
Ponader KC, Charles DF, Belton TJ (2007)Diatom-based TP and TN inference models and indices for monitoring nutrient enrichment of New Jersey streams. Ecol Indic 79:79–93. https://doi.org/10.1016/j.ecolind.2005.10.003
Reynolds CS, Oliver RL, Walsby AE (1987) Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. N Z J Mar Freshw Res 21:379–390. https://doi.org/10.1080/00288330.1987.9516234
Reynolds CS, Lund JWG (1988) The phytoplankton of an enriched, soft-water lake subject to intermittent hydraulic flushing (Grasmere, English Lake District). Freshw Biol 19:379–404. https://doi.org/10.1111/j.1365-2427.1988.tb00359.x
Reynolds CS, Padisák J, Sommer U (1993) Intermediate disturbance in the ecology of phytoplankton and the maintenance of species diversity: a synthesis. In: Padisák J, Reynolds CS, Sommer U (eds) Intermediate disturbance hypothesis in phytoplankton ecology, Proceedings of the 8th workshop of the International Association of Phytoplankton Taxonomy and Ecology 1991, Baja, Hungary, Springer, Dordrecht, pp 183–188
Reynolds CS, Huszar V, Kruk C, Naselli-Flores L, Melo S (2002) Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24:17–428. https://doi.org/10.1093/plankt/24.5.417
Rivier IK (2005) Composition and some ecological features of winter zooplankton in deep stratified lakes. Russ J Ecol 36:179–192. https://doi.org/10.1007/s11184-005-0057-3
Rodrigo MA, Armengol-Díaz X, Oltra R, Dasí MJ, Colom W (2001) Environmental variables and planktonic communities in two ponds of El Hondo wetland (SE Spain). Int Rev Hydrobiol 86:299–315.
Rodrigo MA, Rojo C, Armengol X (2003) Plankton biodiversity in a landscape of shallow water bodies (Mediterranean coast, Spain). Hydrobiologia 506:317–326. https://doi.org/10.1023/B:HYDR.0000008578.62194.04
Rudescu L (1960) Rotatoria. Fauna R.P.Române. Trochelminthes 2(2). Editura Academiei Române, București
Shaw PJ (2003) Canonical correspondence analysis (CCA). In: Arnold H (ed) Multivariate statistics for the environmental sciences. Oxford University Press, New York, pp 232–247
Simpson GL, Oksanen J (2016) analogue: analogue matching and Modern Analogue Technique transfer function models. (R package version 0.17–0). Available at: http://cran.r-project.org/package=analogue (accessed March, 2019)
ter Braak CJF, Smilauer P (1998) CANOCO reference. Manual and user’s guide to Canoco for windows: software for canonical community ordination (version 4). Microcomputer power, Ithaca, NY, USA
Tockner K, Uehlinger U, Robinson TC (2008) Rivers of Europe, First edn. Academic Press, New York
Voight M (1956) Rotatoria – the rotifers of Central Europe. Borntraeger Brothers, Berlin – Nikolasse
Winter JG, Duthie HC (2000) Epilithic diatoms as indicators of stream total N and total P concentration. J North Am Benthol Soc 19:32–49. https://doi.org/10.2307/1468280
Wu N, Schmalz B, Fohrer N (2011) Distribution of phytoplankton in a German lowland river in relation to environmental factors. J Plankton Res 33:807–820. https://doi.org/10.1093/plankt/fbq139
Yin L, Ji Y, Zhang Y, Chong L, Chen L (2018) Rotifer community structure and its response to environmental factors in the backshore wetland of expo garden, Shanghai. Aquaculture and Fisheries 3:90–97. https://doi.org/10.1016/j.aaf.2017.11.001
Zhao SQ, Fang JY, Ji W (2003) Lake restoration from impoldering: impact of land conversion on riparian landscape in Honghu Lake area, Central Yangtze. Agric Ecosyst Environ 95:111–115. https://doi.org/10.1016/S0167-8809(02)00098-1
Zinevici V, Parpală L (2006) The zooplankton structure and productivity in Danube Delta lacustrine ecosystems. In: Tudorancea C, Tudorancea MM (eds) Danube Delta. Genesis and Biodiversity. Backhuys Publishers, Leiden, pp 177–210
Acknowledgments
The study was funded by the Swiss Enlargement Contribution; project IZERZ0 – 142165, “CyanoArchive”, in the framework of the Romanian-Swiss Research Programme and Institute of Biology Bucharest of Romanian Academy; project no. RO1567-IBB02/2013. Octavian Pacioglu was funded by the National Core Program - Romanian Ministry of Research and Innovation Program, project 25 N/2019 BIODIVERS 19270103. The authors thank Stela Sofa for technical support, as well as to the team from the Ecological Station Sulina for assistance during the sampling trips. The authors would equally like to thank two anonymous reviewers that provided us very useful information and checked for scientific inconsistencies and inadvertences along the manuscript.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
Swiss Enlargement Contribution; project IZERZ0–142165, “CyanoArchive”, in the framework of the Romanian-Swiss Research Programme and Institute of Biology Bucharest of Romanian Academy; Project no. RO1567-IBB02/2013 from the Institute of Biology Bucharest of Romanian Academy Octavian Pacioglu was funded by the National Core Program - Romanian Ministry of Research and Innovation Program, project 25 N/2019 BIODIVERS 19270103.
Author information
Authors and Affiliations
Contributions
Larisa I. Florescu, Mirela Moldoveanu and Octavian Pacioglu contributed equally to this work to the conception, analysis and interpretation of data.
Laura Parpala contributed with Cladocera and Copepoda data and revised it critically the article.
All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflicts of interest/competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Online Resource 1
Mean density (cell L−1 for phyto- and individuals L−1 for zooplankton) in the 25 sampled shallow lakes (XLS 91 kb)
Online Resource 2
Mean of physico-chemical parameters per six sampling seasons (in bold the VIF factors), as well as the density of phyto- and zooplankton dominant species (frequency > 1%) employed in statistical analysis (XLS 143 kb)
Rights and permissions
About this article
Cite this article
Florescu, L.I., Moldoveanu, M., Parpală, L. et al. The plankton assemblages as potential bioindicators in the environmental conditions of Danube Delta. Biologia 77, 105–114 (2022). https://doi.org/10.1007/s11756-021-00899-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00899-3