Abstract
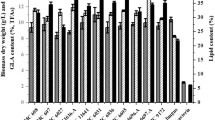
Effects of various cultural conditions on biomass, lipid and Gamma-linolenic acid (GLA) production were investigated in the oleaginous fungus Cunninghamella blakesleeana-JSK2 isolated from soil. The GLA production was influenced by various factors such as growth condition (static and shaken), incubation time, pH, temperature, carbon and nitrogen sources. The results indicated that optimum GLA production (21 %) was obtained when the fungus was grown under shaken condition at 120 rpm for 6 days with optimum pH and temperature of 6 and 28 °C ,respectively. Glucose and potassium nitrate enhanced the GLA production. Urea and sucrose were poor substances for biomass, lipid and GLA production.






Similar content being viewed by others
References
Jantii J, Seppala E, Vapaatalo H, Isomaki H (1989) Evening primrose oil and live oil in treatment of rheumatoid arthritis. Clin Rheumatol 8:238–244
Barber AT (1988) Evening primrose oil: a panacea. Pharmaceut J 240:723–725
Horrobin DF (1979) Schizophrenia: reconciliation of the dopamine, prostaglandin and opioid concepts and the role of the pineal. Lancet 1:529–531
Scott J (1989) Fish and evening primrose oils: gaining medical recognition. Curr Therapeut 45–46
Khoo SK, Munro C, Battisutta D (1990) Evening primrose oil and treatment of premenstrual syndrome. Med J Australia 153:189–192
Ratledge C, Wynn JP (2002) The biochemistry and Molecular biology of lipid accumulation in oleaginous microorganisms. Adv In Appl Microbiol 51:1–51
Fakas S, Makri A, Mavromati M, Tselepi M, Aggelis G (2009) Fatty acid composition in lipid fractions lengthwise the mycelium of Mortierella isabellina and lipid production by solid state fermentation. Biores Tech 100:6118–6120
Fakas S, Papanikolaou S, Batsos A, Galiotou-Panayotou M, Mallouchos A, Aggelis G (2009) Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 33:573–580
Jangbua P, Laoteng K, Kitsubun P, Nopharatana M, Tongta A (2009) Gamma-linolenic acid production of Mucor rouxii by solid-state fermentation using agricultural by-products. Lett In App Microbiol 49:91–97
Kristofikova L, Rosenberg M, Vlnova A (1991) Selection of Rhizopus strains for l(+)-lactic acid and gamma-linolenic acid production. Folia Microbiol (Praha) 36:451–455
Weete JD, Shewmaker F, Gandhi SR (1998) γ-Linolenic acid in zygomycetous fungi: Syzygites megalocarpus. J Am Oil Chem Soc 75:1367–1372
Booth C (1971) Fungal culture media. In: Booth C (ed) Methods in microbiology, vol 4. Academic Press, London
White TJ (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic Press, San Diego, pp 315–322
Somashekar D, Venkateswaran G, Sambaiah K et al (2002) Effect of culture conditions on lipid and gamma-linolenic acid production by mucoraceous fungi. Proc Biochem 38:1719–1724
Folch JM, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Savitha J, Wynn JP, Ratledge C (1997) Malic enzyme: its purification and characterization from Mucor circinelloides and occurrence in other oleaginous fungi. W J Microbiol Biotechnol 13:7–9
Certik M, Slavikova L, Masrnova S et al (2006) Enhancement of nutritional value of cereals with γ-linolenic acid by fungal solid state fermentations. Food Technol Biotechnol 44:75–82
Jang HD, Lin YY, Yang SS (2005) Effect of culture media and conditions on polyunsaturated fatty acids production by Mortierella alpina. Biores Technol 96:1633–1644
Yamada H, Shimizu S, Shinmen Y (1987) Production of arachidonic acid by Mortierella elongata IS-5. Agr Biol Chem 51:785–790
Bajpai PK, Bajpai P (1992) Review: arachidonic acid production by microorganisms. Biotechnol Appl Biochem 15:1–10
Sumner JL, Morgan ED, Evans HC (1969) The effect of temperature on the fatty acid composition of fungi in the order Mucorales. Can J Microbiol 15:515–520
Nisha A, Venkateswaran G (2011) Effect of culture variables on mycelial arachidonic acid production by Mortierella alpine. Food Bioproc Technol 4:232–240
Dyal SD, Bouzidi L, Narine SS (2005) Maximizing the production of γ-linolenic acid in Mortierella ramanniana var. ramanniana as a function of pH, temperature and carbon source, nitrogen source, metal ions and oil supplementation. Food Res Inter 38:815–829
Ahmed SU, Singh SK, Pandey A et al (2006) Effects of various process parameters on the production of γ-linolenic acid in submerged fermentation. Food Technol Biotechnol 44:283–287
Chen HC, Chang CC (1996) Production of γ-linolenic acid by the fungus Cunninghamella echinulata CCRC 31840. Biotechnol Prog 12:338–341
Wynn JP, Hamid AA, Ratledge C et al (2001) Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi Mucor circinelloides and Mortierella alpina. Microbiology 147:2857–2864
Certik M, Megova J, Horenitzky R (1999) Effect of nitrogen sources on the activities of lipogenic enzymes in oleaginous fungus Cunninghamella echinulata. J Gen Appl Microbiol 45:289–293
Acknowledgments
The authors acknowledge the University Grant Council (UGC), India for funding the research project entitled “Lipid profile of endophytic fungi: Identification of suitable strain for the production of commercially important omega fatty acids (EPA & DHA)”. The work was also supported by grant VEGA 1/0975/12 from the Grant Agency of the Ministry of Education, Slovak Republic and by grants APVV-0662-11 and APVV-0294-11 from the Slovak Research and Development Agency, Slovak Republic.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sukrutha, S.K., Adamechova, Z., Rachana, K. et al. Optimization of Physiological Growth Conditions for Maximal Gamma-linolenic Acid Production by Cunninghamella blakesleeana-JSK2. J Am Oil Chem Soc 91, 1507–1513 (2014). https://doi.org/10.1007/s11746-014-2507-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2507-1