Abstract
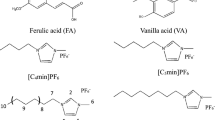
Mono glyceryl ferulate (MFG), an all-natural derivative of ferulic acid, can be used as antioxidant and a UV filter in food and cosmetic formulations. However, the applications of MFG in these fields are limited due to its low availability in nature. In this work, a novel method for selective separation of MFG from a room temperature ionic liquid (RTIL) solution of enzymatic transesterification of ethyl ferulate with glycerol was investigated. The effects of different extractants and extraction conditions on the selective separation of MFG were also studied. High extraction yields of MFG (98.23 ± 1.09 %) and the relative content of MFG in the extracts (97.76 ± 1.25 %) were achieved using neutral deionized water as extractant under the following conditions: 0.5:1 volume ratio of water to RTIL solution, extraction time of 6 min, centrifugation time of 6 min, and extraction done eight times. The extraction thermodynamics showed that the extraction of MFG was an exothermic process. The purified MFG exhibited good UV adsorbing properties at 280–360 nm with a λ max at 322 nm.






Similar content being viewed by others
References
Compton DL (2005) Sunscreens based on vegetable oil. Lipid Technol 17:276–279
Compton DL, Laszlo JA, Evans KO (2012) Antioxidant properties of feruloyl glycerol derivatives. Ind Crops Prod 36:217–221
Laszlo JA, Evans KO, Vermillion KE, Appell M (2010) Feruloyl dioleoylglycerol antioxidant capacity in phospholipid vesicles. J Agric Food Chem 58:5842–5850
Dobberstein D, Bunzel M (2010) Separation and detection of cell wall-bound ferulic acid dehydrodimers and dehydrotrimers in cereals and other plant materials by reversed phase high-performance liquid chromatography with ultraviolet detection. J Agric Food Chem 58:8927–8935
Graça J, Pereira H (2000) Suberin structure in potato periderm: glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J Agric Food Chem 48:5476–5483
Matsuo T, Kobayashi T, Kimura Y, Tsuchiyama M, Oh T, Sakamoto T, Adachi S (2008) Synthesis of glyceryl ferulate by immobilized ferulic acid esterase. Biotechnol Lett 30:2151–2156
Matsuo T, Kobayashi T, Kimura Y, Hosoda A, Taniguchi H, Adachi S (2008) Continuous synthesis of glyceryl ferulate using immobilized Candida antarctica lipase. J Oleo Sci 57:375–380
Tsuchiyama M, Sakamoto T, Fujita T, Murata S, Kawasaki H (2006) Esterification of ferulic acid with polyols using a ferulic acid esterase from Aspergillus niger. BBA-Gen Subj 1760:1071–1079
Sun S, Shan L, Jin Q, Liu Y, Wang X (2007) Solvent-free synthesis of glyceryl ferulate using a commercial microbial lipase. Biotechnol Lett 29:945–949
Sun S, Yang G, Bi Y, Xiao F (2009) Chemoenzymatic synthesis of feruloylated monoacyl-and diacyl-glycerols in ionic liquids. Biotechnol Lett 31:1885–1889
Sun S, Song F, Bi Y, Yang G, Liu W (2012) Solvent-free enzymatic transesterification of ethyl ferulate and monostearin: optimized by responce surface methodology. J Biotechnol 164:340–345
Sun S, Shan L, Liu Y, Jin Q, Zhang L, Wang X (2008) Solvent-free enzymatic preparation of feruloylated monoacylglycerols optimized by response surface methodology. J Agric Food Chem 56:442–447
Sun S, Qin F, Bi Y, Chen J, Yang G, Liu W (2013) Enhanced transesterification of ethyl ferulate with glycerol for preparing glyceryl diferulate using a lipase in ionic liquids as reaction medium. Biotechnol Lett 35:1449–1454
Baker GA, Baker SN, Pandey S, Bright FV (2005) An analytical view of ionic liquids. Analyst 130:800–808
Hardacre C, Holbrey JD, Nieuwenhuyzen M, Youngs TGA (2007) Structure and Solvation in Ionic Liquids. Acc Chem Res 40:1146–1155
Han X, Armstrong DW (2007) Ionic liquids in separations. Acc Chem Res 40:1079–1086
Fu D, Mazza G, Tamaki Y (2010) Lignin extraction from straw by ionic liquids and enzymatic hydrolysis of the cellulosic residues. J Agric Food Chem 58:2915–2922
Liang R, Bao Z, Su B, Xing H, Yang Q, Yang Y, Ren Q (2013) Feasibility of ionic liquids as extractants for selective separation of Vitamin D3 and Tachysterol3 by solvent extraction. J Agric Food Chem 61:3479–3487
Gorri D, Ruiz A, Ortiz A, Ortiz I (2009) The use of ionic liquids as efficient extraction medium in the reactive separation of cycloolefins from cyclohexane. Chem Eng J 154:241–245
Chen D, OuYang X, Wang Y, Yang L, He C (2013) Liquid–liquid extraction of caprolactam from water using room temperature ionic liquids. Sep Purif Technol 104:263–267
Berthod A, Ruiz-Ángel MJ, Carda-Broch S (2008) Ionic liquids in separation techniques. J Chromatogr A 1184:6–18
Yang Q, Xing H, Su B, Yu K, Bao Z, Yang Y, Ren Q (2012) Improved separation efficiency using ionic liquid–cosolvent mixtures as the extractant in liquid–liquid extraction: a multiple adjustment and synergistic effect. Chem Eng J 181–182:334–342
Cao Y, Wu J, Zhang J, Li H, Zhang Y, He J (2009) Room temperature ionic liquids (RTILs): a new and versatile platform for cellulose processing and derivatization. Chem Eng J 147:13–21
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94:2319–2358
Fletcher KA, Storey IA, Hendricks AE, Pandey S, Pandey S (2001) Behavior of the solvatochromic probes Reichardt’s dye, pyrene, dansylamide, Nile Red and 1-pyrenecarbaldehyde within the room-temperature ionic liquid bmimPF6. Green Chem 3:210–215
Naczk M, Shahidi F (2004) Extraction and analysis of phenolics in food. J Chromatogr A 1054:95–111
Spelzini D, Farruggia B, Picó G (2005) Features of the acid protease partition in aqueous two-phase systems of polyethylene glycol–phosphate: chymosin and pepsin. J Chromatogr B 821:60–66
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (31101301), Funding Scheme for Young Teachers in Colleges and Universities in Henan Province (2012GGJS-83), and the Plan for Scientific Innovation Talent of Henan University of Technology (11CXRC03).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sun, S., Chen, X., Bi, Y. et al. Selective Separation of Mono Glyceryl Ferulate Using Water from an Ionic Liquid Solution of Enzymatic Transesterification. J Am Oil Chem Soc 91, 1339–1345 (2014). https://doi.org/10.1007/s11746-014-2491-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2491-5