Abstract
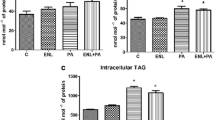
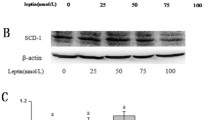
Trans fatty acids (TFA) intake has been linked to cardiovascular diseases and liver diseases; yet the effect of TFA on inflammation remains controversial. Accordingly, the objective of this paper was to determine the in vitro effects of TFA on inflammatory gene expression. Human umbilical vein endothelial cells (HUVEC) and human hepatocellular carcinoma (HepG2) cells were treated for 24 h with either trans-vaccenic acid (tVA), trans-palmitoleic acid (tPA) or elaidic acid (EA) at concentrations of 5–150 µM, or with a mixture of tVA and tPA (150/50 µM). All TFA were highly incorporated into cell membranes, as determined by gas chromatography, representing 15–20% of total fatty acids in HUVEC and 3–8% in HepG2 cells. Incorporation of EA, a common industrial TFA, increased the ratio of the stearoyl-CoA desaturase (SCD-1), a key enzyme involved in fatty acid metabolism. Ruminant TFA, including tVA, tPA and the mixture of tVA and tPA, significantly reduced the TNF-α-induced gene expression of TNF, VCAM-1 and SOD2 in HUVEC, as well as TNF and IL-8 in HepG2 cells. EA also decreased inflammatory gene expression in HUVEC, but not in HepG2 cells. The inhibition of peroxisome proliferator-activated receptor (PPAR)-γ did not influence the effects of TFA on gene expression. Overall, physiological and supraphysiological concentrations of TFA, especially tVA and tPA, prevented inflammatory gene expression in vitro. This effect is independent of PPAR-γ activation and may be due to an alteration of fatty acid metabolism in cell membranes caused by the high incorporation of TFA.







Similar content being viewed by others
Abbreviations
- CVD:
-
Cardiovascular disease
- DMSO:
-
Dimethyl sulfoxide
- EA:
-
Elaidic acid
- eNOS:
-
Endothelial nitric oxide synthase
- HepG2:
-
Liver hepatocellular cells
- HUVEC:
-
Human umbilical vein endothelial cells
- IL:
-
Interleukin
- iTFA:
-
Industrial TFA
- LC–MS/MS:
-
Liquid chromatography coupled with tandem mass spectrometry
- NF-κB:
-
Nuclear factor κB
- NO:
-
Nitric oxide
- PG:
-
Prostaglandin
- PPAR:
-
Peroxisome proliferator-activated receptor
- rTFA:
-
Ruminant TFA
- SOD:
-
Superoxide dismutase
- TFA:
-
Trans fatty acids
- TNF-α:
-
Tumor necrosis factor alpha
- tPA:
-
trans-Palmitoleic acid
- tVA:
-
trans-Vaccenic acid
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116:1793–1801. doi:10.1172/JCI29069
Rocha VZ, Libby P (2009) Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 6:399–409. doi:10.1038/nrcardio.2009.55
Park EJ, Lee JH, Yu GY et al (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140:197–208. doi:10.1016/j.cell.2009.12.052
Landmesser U, Hornig B, Drexler H (2004) Endothelial function: a critical determinant in atherosclerosis? Circulation 109:II27–II33. doi:10.1161/01.CIR.0000129501.88485.1f
Rajendran P, Rengarajan T, Thangavel J et al (2013) The vascular endothelium and human diseases. Int J Biol Sci 9:1057–1069. doi:10.7150/ijbs.7502
Sun B, Karin M (2012) Obesity, inflammation, and liver cancer. J Hepatol 56:704–713. doi:10.1016/j.jhep.2011.09.020
Calder PC, Ahluwalia N, Albers R et al (2013) A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr 109:S1–S34. doi:10.1017/S0007114512005119
Mozaffarian D, Katan MB, Ascherio A et al (2006) Trans fatty acids and cardiovascular disease. New Engl J Med 354:1601–1613
Månsson HL (2008) Fatty acids in bovine milk fat. Food Nutr Res 52:1–3. doi:10.3402/fnr.v52i0.1821
Bassett CMC, Edel AL, Patenaude AF et al (2010) Dietary vaccenic acid has antiatherogenic effects in LDLr−/− mice. J Nutr 140:18–24. doi:10.3945/jn.109.105163
Dhibi M, Brahmi F, Mnari A et al (2011) The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr Metab (Lond) 8:65. doi:10.1186/1743-7075-8-65
Tardy A-L, Morio B, Chardigny J-M, Malpuech-Brugère C (2011) Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases. Nutr Res Rev 24:111–117. doi:10.1017/S0954422411000011
Harvey KA, Walker CL, Xu Z et al (2012) Trans fatty acids: induction of a pro-inflammatory phenotype in endothelial cells. Lipids 47:647–657. doi:10.1007/s11745-012-3681-2
Iwata NG, Pham M, Rizzo NO et al (2011) Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS One 6:e29600. doi:10.1371/journal.pone.0029600
Bryk D, Malecki M, Hajdukiewicz K, Sitkiewicz D (2011) Trans fatty acids induce a proinflammatory response in endothelial cells through ROS-dependent nuclear factor-κB activation. J Physiol Pharmacol 62:229–238
Jaudszus A, Jahreis G, Schlörmann W et al (2012) Vaccenic acid-mediated reduction in cytokine production is independent of c9, t11-CLA in human peripheral blood mononuclear cells. Biochim Biophys Acta Mol Cell Biol Lipids 1821:1316–1322. doi:10.1016/j.bbalip.2012.06.010
Livingstone KM, Givens DI, Jackson KG, Lovegrove JA (2014) Comparative effect of dairy fatty acids on cell adhesion molecules, nitric oxide and relative gene expression in healthy and diabetic human aortic endothelial cells. Atherosclerosis 234:65–72. doi:10.1016/j.atherosclerosis.2014.02.015
Ricciotti E, Fitzgerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31:986–1000. doi:10.1161/ATVBAHA.110.207449
Da Silva MS, Rudkowska I (2015) Dairy nutrients and their effect on inflammatory profile in molecular studies. Mol Nutr Food Res 59:1249–1263. doi:10.1002/mnfr.201400569
Vesper H, Kuiper H, Mirel L et al (2012) Levels of plasma trans-fatty Acids in non-hispanic white adults in the United States in 2000 and 2009. JAMA 307:562–563
Potenza MA, Addabbo F, Montagnani M (2009) Vascular actions of insulin with implications for endothelial dysfunction. Am J Physiol Endocrinol Metab 297:E568–E577. doi:10.1152/ajpendo.00297.2009
Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25:29–38. doi:10.1161/01.ATV.0000150649.39934.13
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Larose J, Julien P, Greffard K et al (2014) F2-isoprostanes are correlated with trans fatty acids in the plasma of pregnant women. Prostaglandins Leukot Essent Fatty Acids 91:243–249. doi:10.1016/j.plefa.2014.09.010
Shaikh NA, Downar E (1981) Time course of changes in porcine myocardial phospholipid levels during ischemia. A reassessment of the lysolipid hypothesis. Circ Res 49:316–325. doi:10.1161/01.RES.49.2.316
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Da Silva MS, Julien P, Pérusse L et al (2015) Natural rumen-derived trans fatty acids are associated with metabolic markers of cardiac health. Lipids 50:873–882. doi:10.1007/s11745-015-4055-3
Kumar A, Takada Y, Boriek AM, Aggarwal BB (2004) Nuclear factor-kappaB: its role in health and disease. J Mol Med 82:434–448. doi:10.1007/s00109-004-0555-y
Lee KS, Kim J, Kwak SN et al (2014) Functional role of NF-κB in expression of human endothelial nitric oxide synthase. Biochem Biophys Res Commun 448:101–107. doi:10.1016/j.bbrc.2014.04.079
Daynes RA, Jones DC (2002) Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol 2:748–759. doi:10.1038/nri912
Kliewer SA, Sundseth SS, Jones SA et al (1997) Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 94:4318–4323
Wang Y, Jacome-Sosa MM, Ruth MR et al (2012) The intestinal bioavailability of vaccenic acid and activation of peroxisome proliferator-activated receptor-alpha and -gamma in a rodent model of dyslipidemia and the metabolic syndrome. Mol Nutr Food Res 56:1234–1246. doi:10.1002/mnfr.201100517
Turk HF, Chapkin RS (2013) Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fat Acids 88:43–47. doi:10.1016/j.plefa.2012.03.008
Ganguly R, LaVallee R, Maddaford TG et al (2016) Ruminant and industrial trans fatty acid uptake in the heart. J Nutr Biochem 31:60–66. doi:10.1016/j.jnutbio.2015.12.018
Woldseth B, Retterstøl K, Christophersen BO (1998) Monounsaturated trans fatty acids, elaidic acid and trans-vaccenic acid, metabolism and incorporation in phospholipid molecular species in hepatocytes. Scand J Clin Lab Invest 58:635–645. doi:10.1080/00365519850186067
Pan Y, Liu B, Deng Z et al (2017) Lipid rafts promote trans fatty acid-induced inflammation in human umbilical vein endothelial cells. Lipids 52:27–35. doi:10.1007/s11745-016-4213-2
Smit LA, Katan MB, Wanders AJ et al (2011) A High intake of trans fatty acids has little effect on markers of inflammation and oxidative stress in humans. J Nutr 141:1673–1678. doi:10.3945/jn.110.134668
Bendsen NT, Stender S, Szecsi PB et al (2011) Effect of industrially produced trans fat on markers of systemic inflammation: evidence from a randomized trial in women. J Lipid Res 52:1821–1828. doi:10.1194/jlr.M014738
Lacroix E, Couillard C, Paquin P et al (2010) Effect of trans fatty acids from ruminants and industrial sources on plasma adhesion molecule concentrations in healthy men. FASEB J 24(724):16
Brouwer IA, Wanders AJ, Katan MB (2010) Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans–a quantitative review. PLoS One 5:e9434. doi:10.1371/journal.pone.0009434
Vendel Nielsen L, Krogager TP, Young C et al (2013) Effects of elaidic acid on lipid metabolism in hepg2 cells, investigated by an integrated approach of lipidomics, transcriptomics and proteomics. PLoS One 8:e74283. doi:10.1371/journal.pone.0074283
Minville-Walz M, Gresti J, Pichon L et al (2012) Distinct regulation of stearoyl-CoA desaturase 1 gene expression by cis and trans C18:1 fatty acids in human aortic smooth muscle cells. Genes Nutr 7:209–216. doi:10.1007/s12263-011-0258-2
Krogager TP, Nielsen LV, Kahveci D et al (2015) Hepatocytes respond differently to major dietary trans fatty acid isomers, elaidic acid and trans-vaccenic acid. Proteome Sci 13:31. doi:10.1186/s12953-015-0084-3
Alstrup KK, Brock B, Hermansen K (2004) Long-term exposure of INS-1 cells to cis and trans fatty acids influences insulin release and fatty acid oxidation differentially. Metabolism 53:1158–1165. doi:10.1016/j.metabol.2004.02.026
Du ZY, Degrace P, Gresti J et al (2010) Dissimilar properties of vaccenic versus elaidic acid in β-oxidation activities and gene regulation in rat liver cells. Lipids 45:581–591. doi:10.1007/s11745-010-3428-x
Jaudszus A, Kramer R, Pfeuffer M et al (2014) trans Palmitoleic acid arises endogenously from dietary vaccenic acid. Am J Clin Nutr 99:431–435. doi:10.3945/ajcn.113.076117
Kratz M, Marcovina S, Nelson JE et al (2014) Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not beta-cell function in humans. Am J Clin Nutr 99:1385–1396. doi:10.3945/ajcn.113.075457
Mozaffarian D, Cao H, King IB et al (2010) trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S Adults. Ann Intern Med 153:790–799. doi:10.7326/0003-4819-153-12-201012210-00005
Nestel PJ, Straznicky N, Mellett NA et al (2014) Specific plasma lipid classes and phospholipid fatty acids indicative of dairy food consumption associate with insulin sensitivity. Am J Clin Nutr 99:46–53. doi:10.3945/ajcn.113.071712
Bordoni A, Danesi F, Dardevet D et al (2015) Dairy products and inflammation: a review of the clinical evidence. Crit Rev Food Sci Nutr. doi:10.1080/10408398.2014.967385
Acknowledgements
The authors would like to thank Line Berthiaume, who performed gas chromatographic analyses to determine fatty acid profiles, as well as Jessica Larose and Karine Greffard for the determination of PGE2 levels by LC–MS/MS. The authors also thank Mélanie Verreault for useful assistance with the HepG2 cell culture. This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC). M.S.D.S. has received scholarships from the Centre de recherche en endocrinologie moléculaire et oncologique et génomique humaine (CREMOGH), the department of kinesiology of Université Laval and the CHU de Québec Foundation—Desjardins. I.R. holds a Junior 1 Research Scholar award from the Fonds de Recherche du Québec—Santé (FRQ-S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
About this article
Cite this article
Da Silva, M.S., Julien, P., Bilodeau, JF. et al. Trans Fatty Acids Suppress TNF-α-Induced Inflammatory Gene Expression in Endothelial (HUVEC) and Hepatocellular Carcinoma (HepG2) Cells. Lipids 52, 315–325 (2017). https://doi.org/10.1007/s11745-017-4243-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4243-4