Abstract
The term non-alcoholic fatty liver disease (NAFLD) has rapidly become the most common type of chronic liver disease. NAFLD points to excessive hepatic fat storage and no evidence of secondary hepatic fat accumulation in patients with “no or little alcohol consumption”. Both the etiology and pathogenesis of NAFLD are largely unknown, and a definitive therapy is lacking. Since NAFLD is very often and closely associated with metabolic dysfunctions, a consensus process is ongoing to shift the acronym NAFLD to MAFLD, i.e., metabolic-associated fatty liver disease. The change in terminology is likely to improve the classification of affected individuals, the disease awareness, the comprehension of the terminology and pathophysiological aspects involved, and the choice of more personalized therapeutic approaches while avoiding the intrinsic stigmatization due to the term “non-alcoholic”. Even more recently, other sub-classifications have been proposed to concentrate the heterogeneous causes of fatty liver disease under one umbrella. While awaiting additional validation studies in this field, we discuss the main reasons underlying this important shift of paradigm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The acronyms NASH (non-alcoholic steatohepatitis) and NAFLD (non-alcoholic fatty liver disease) were originally coined by Ludwig et al. in 1980 [1] and Shaffner and Thaler in 1986 [2], respectively. NAFLD is a clinico-histopathologic entity defining excessive hepatic fat storage without evidence of secondary hepatic fat accumulation [3,4,5,6,7] in patients with “no or little alcohol consumption”. Hepatic fat content must be greater than 5% at liver histology [8] or 5.6% at magnetic resonance [9].
Since 2020, a debate is taking place worldwide to redefine NAFLD as metabolic (dysfunction)-associated fatty liver disease (MAFLD), according to the most prevalent causes of liver steatosis [10,11,12]. Meantime, further terminologies are being proposed instead of NAFLD. While awaiting additional validation studies, the main reasons underlying this important shift of paradigm are discussed in the following paragraphs.
NAFLD and the burden of disease: prevalence, natural history, etiology and pathogenesis
Globally, NAFLD has reached epidemic levels, with pooled prevalence of 14% (Africa), 24% (North America) [13], 24–27% (range 18–40%, Europe) [14], 27% (Asia), 31% (South America), and 32% (Middle East) [13]. NAFLD is more frequent in men than in women (33 vs. 20%), and also affects 10–20% of the pediatric population [15]. Evidence indicates the prevalence of NAFLD is on the rise worldwide together with that of obesity and associated complications [11, 16, 17].
In this scenario, we can expect a time-dependent increase of the incidence of liver fibrosis, decompensated liver cirrhosis, hepatocellular carcinoma, and liver-related mortality due to the progressive deterioration of initial NAFLD in the affected population [17].
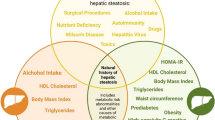
The natural history of NAFLD can vary, with most patients developing a benign or slowly progressive form, which is usually asymptomatic in the early stages. The spectrum ranges from simple steatosis (non-alcoholic fatty liver, NAFL) to the progressive non-alcoholic steatohepatitis (NASH) in about 20% of cases [18]. NASH has the potential to progress to compensated and decompensated liver cirrhosis and, with more than a tenfold increase in risk, to hepatocellular carcinoma (HCC) (Fig. 1) [9, 19]. Of note, NAFLD is also a risk factor for several extrahepatic, especially metabolic manifestations [20] and is linked with increased cardiovascular risk [21].
Causal factors, protective factors and the continuum spectrum of natural history of non-alcoholic fatty liver disease (NAFLD). Factors on the left have an established association with NAFLD and NASH progression. They are broadly classified into genetic factors, comorbid illness, and environmental factors. On the right, factors have a protective role. F0–F4, fibrosis scores (potentially reversible)
Excessive liver fat storage has a series of well-known causes (Supplementary Table 1) and originates from a dynamic balance between causal and protective factors (Fig. 1) [22, 23].
In this scenario, individual lifestyle changes and environmental factors can promote epigenetic mechanisms such as histone methylation, abnormal DNA methylation, miRNA profiles, able to affect gene expression and to influence the progression of disease. Examples are the consequences of metabolic disturbances in pregnancy on NAFLD offspring [24], and the pro-inflammatory liver response and weight gain in germ-free mice colonized with stool microbes from 2-week-old infants born to obese mothers [25]. These mechanisms interact with inherited risk factors and modulate individual susceptibility to NAFLD [26].
Lifestyle and dietary habits [27, 28] play a key role, and NAFLD is commonly associated with metabolic abnormalities [9] such as obesity, type 2 diabetes, dyslipidemia, hypertension, hypopituitarism, and sedentary life [20]. On the other hand, alcohol, air pollution [29, 30], food contaminants [31], and gut dysbiosis, e.g., higher proportion of Proteobacteria and E. coli, with a lower proportion of Firmicutes, especially F. prausnitzii [32] likely contribute to the onset and progression of NAFLD [20, 33, 34].
In the context of metabolic abnormalities, several pathogenic pathways are involved in lipotoxicity and can contribute to the onset and progression of NAFLD, according to the nomenclature in use. The “lean” healthy visceral adipose tissue expresses anti-inflammatory cytokines (i.e., adiponectin, interleukin IL-4, IL-10, IL-13, transforming growth factor (TGF)-β, and nitric oxide (NO)) which control M2 macrophagic response and inhibit the neutrophil-mediated inflammation. By contrast, during expansion of hypertrophic (and apoptotic) visceral adipose tissue, secretion of pro-inflammatory molecules such as leptin, resistin, IL-6 and tumor necrosis factor (TNF)-α occurs. This step activates a M1 macrophage response [35], and results in insulin resistance, a chronic “metabolic” inflammatory status, and increased lipolysis of triglycerides with abundant flux of blood long-chain free fatty acids (FFA) to the liver. In addition, FFA in blood increases because of fat-enriched dietary habits and from dietary sugars driving the de novo lipogenesis (DNL) in the liver. Altogether, these factors contribute to the expansion of the hepatocyte FFA pool which can stress the mitochondrial β-oxidation capacity, lead to defective secretion/export of very-low density lipoproteins (VLDL) to blood. Accumulation of lipotoxic species such as lysophosphatidylcholine, diacylglycerol, and ceramides mediates the endoplasmic reticulum (ER) stress, the cellular oxidative stress, and the activation of the inflammasome. This is a component of the innate immunity response consisting of a multiprotein cytoplasmic complex activated by several damage-associated molecular patterns (DAMPs). Additional abnormalities consist of dysregulation of adipocytokines, depleted mitochondrial ATP, production of toxic uric acid, periodic hypoxia (i.e., during sleep apnea in extremely obese patients), and toxic products from gut microbiome which include tumor necrosis factor (TNF)-α, endogenous ethanol, and endotoxins like lipopolysaccharides (LPS). Studies in pure fatty liver models without fibrosis suggest that lipid accumulation developing with obesity can induce a distortion of liver architecture manifesting with reduced sinusoidal space and increased intrahepatic vascular resistance. Such changes can pave the way to portal hypertension observed in obesity, and progression to hemodynamically decompensated liver cirrhosis [36,37,38,39].
Conditions can promote the NASH phenotype which manifests with hepatocellular injury, inflammation, stellate cell activation and progressive accumulation of excess extracellular matrix. Additional targets of the ongoing cellular damage include intracellular organelles, the nucleus, receptors and signaling pathways [40,41,42,43,44].
NAFLD and metabolic dysfunctions
As mentioned earlier, the diagnosis of NAFLD is based on hepatic steatosis at imaging techniques or histology, and “exclusion” of competing causes of liver disease including “significant” alcohol intake. Such strict definition has some limitations. Despite the NAFLD prognosis depends on the presence of fibrotic NASH, the ultimate utility of liver biopsy becomes questionable [45]. In fact, liver biopsy is invasive, painful, risky, prone to misclassification due to sampling errors, not easily performed in large groups of patients who still lack targeted therapies for NAFLD. The issue of alcohol intake in NAFLD deserves additional observations. A standard drink contains about 14 g of pure alcohol (Rethinking Drinking Homepage—NIAAA (nih.gov)) and the current definition for NAFLD must exclude a weekly intake of ≥ 21 and ≥ 14 drinks in males and females, respectively. Above this cutoff value, the risk of alcoholic fatty liver disease (ALD) increases [46, 47], but it is difficult to exactly calculate the intake of alcoholic units or the duration of alcohol abstinence. Phosphatidylethanol can become a potential biomarker of alcohol consumption [48]. Yet, the effect of modest alcohol intake at lower cutoff values is still controversial in NAFLD individuals [49, 50].
In addition, the diagnosis of NAFLD requires the exclusion of several other causes of liver steatosis such as viral hepatitis [51,52,53,54], hepatotoxic drugs [55], Wilson’s disease [56], total parenteral nutrition, prolonged fasting [57], and several other less common conditions (Supplementary Table 1). Both NAFLD and ALD rank as the most frequent conditions [58]. On one hand, the diagnosis of NAFLD relies on exclusion criteria and does not require the presence of metabolic dysfunction. But NAFLD is no longer an isolated condition, since NAFLD is associated with morbid obesity in about 90% of the cases [59, 60], with obesity and dyslipidemia in over 80% of the cases [61, 62], with hypertension in 70% of cases and with type 2 diabetes (T2DM) in about 50% of the cases [63,64,65]. Such close associations strongly suggests that NAFLD is a systemic disease [10, 11, 15, 66] increasing with poor lifestyles, and in parallel with the epidemiological raise of overweight, obesity, insulin resistance, and metabolic syndrome [4, 12, 67,68,69,70]. Younossi et al. [71] studied the prevalence of NAFLD and NASH in a metanalysis including 80 studies from 20 countries worldwide and 49,419 patients with T2DM. The prevalence of NAFLD was more than twofold higher than in the general population, i.e., 56%. In the same group, the prevalence of NASH was 37%. In patients with NAFLD and T2DM undergoing liver biopsy, 17% had advanced (F3–F4) liver fibrosis [71]. The close association of NAFLD with systemic metabolic conditions also explains why cardiovascular complications are frequent in NAFLD patients [65, 72], including atrial fibrillation [73, 74], diabetes, chronic kidney disease and extrahepatic neoplasms [75,76,77]. It is worth noting that 10–30% of non-obese individuals can have NAFLD [4, 78].
Another close link between NAFLD and metabolic dysfunction is that the metabolic syndrome is often associated with NAFLD and with increased cardiovascular disease. NAFLD per se, however, is independently associated with cardiovascular disease [65, 72, 79, 80].
The ultimate knowledge of complex mechanisms governing the onset and progression of NAFLD is poorly known. The term “steatosis” is intrinsically characterized by the interplay of multifactorial factors [34, 40, 81, 82]. The coexistence of such factors makes the diagnosis of NAFLD and the design of clinical trials often difficult, since several confounding factors can be present [9].
MAFLD: debate about nomenclature
The acronym NAFLD was originally established as a diagnosis of exclusion based on the use of the stigmatizing term “alcoholic” and on a poor pathophysiological knowledge. In the past years, this term has generated confusion or uncertainty with regard to knowledge and allocation in clinical trials [83, 84]. Studies show that up to 96% of subjects with NAFLD can be unaware they have liver disease [85].
Following the current directions, there is a need for adopting a better term for liver steatosis to emphasize what the disease “is”, rather than what “it is not” when considering the burden of contributing metabolic abnormalities, pathophysiological mechanisms, diagnostic, and therapeutic strategies [84]. Since 2020 experts from 134 countries have proposed the transition of terminology from NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) [10] which points to the close association between fatty liver, metabolic disorders and target organ dysfunction (i.e., diabetes, chronic kidney disease, atherosclerosis, lung dysfunction, colon cancer, and both intrahepatic and extrahepatic events) [86], rather than on exclusion criteria [12]. The new terminology is not yet endorsed by international societies, including European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Disease AASLD) [87]. MAFLD is based on the presence of fatty liver at imaging, or by the combination of serum biomarkers or liver biopsy (Table 1) in individuals with overweight/obesity (different in Caucasians or Asians), T2DM (i.e., HbA1c ≥ 6.5% or specific drug treatment). In lean/normal weight individuals, the diagnosis is based on the presence of metabolic dysregulation with at least two out of seven abnormalities which include increased waist circumference, blood pressure, hypertriglyceridemia, low plasma high-density lipoprotein cholesterol, impaired fasting plasma glucose, insulin resistance with impaired Homeostatic Model Assessment of Insulin Resistance, and subclinical inflammation by plasma high‐sensitivity C‐reactive protein level [10, 83, 88,89,90,91,92] (Fig. 2A).
A The flowchart depicts the essential steps involved in the positive diagnosis of MAFLD vs. the diagnosis of NAFLD which requires the exclusion of secondary causes. Adapted from [10, 134]. B Exclusive and overlapping features in the spectrum of definitions ranging from non‐alcoholic fatty liver disease (NAFLD) to metabolic-dysfunction‐associated fatty liver disease (MAFLD). Significant alcohol intake is ≥ 30 g/day and ≥ 20 g/day in men and women, respectively
According to this perspective, the adoption of MAFLD definition provides a better classification of patients at higher risk of cardiovascular and kidney diseases [21, 93,94,95], and hepatic fibrosis [96,97,98], independently of other causes of liver damage. For example, MAFLD terminology becomes independent of alcohol intake, a situation which appears to worsen hepatic fibrosis and increase mortality in liver steatosis [99,100,101,102,103]. On the other hand, one could argue that liver disease in general is often associated with alcohol abuse in the perception of the general public, and that adding “non-alcoholic” would be beneficial since it is now explicitly stated that the liver disease is not related to alcohol.
The presence of viral hepatitis is not an “a priori” exclusion criterium for MAFLD. In addition, MAFLD represents a further risk factor for HCC in hepatitis C virus (HCV) and hepatitis B virus (HBV) patients [104, 105]. Vice versa, the 10-year risk of cardiovascular disease is higher in patients with MAFLD and concomitant viral infection by hepatitis B or C virus, than in patients with only MAFLD [106]. With MAFLD, moreover, the role of metabolic abnormalities on liver damage will be elaborated together with other causes of liver steatosis, such as drugs, pregnancy and gut surgery [107,108,109]. This possibility will be labeled as “MAFLD plus additional cause ….” [11, 96].
The diagnosis of MAFLD can include the presence of positive biomarkers independently from imaging and histology. This is not the case in NAFLD (see above). Thus, the possibility exists that a patient referred with a high-score for a specific algorithm such as the fatty liver index [110]) will be classified as MAFLD in the presence of metabolic dysfunction.
By contrast, the criteria designed for MAFLD do not extend to individuals with liver steatosis without metabolic dysfunction.
In a recent metanalysis and systematic review involving a pool of over 3 million individuals, the prevalence of MAFLD was 39%, 30%, and 5% in obese, non-obese, and normal weight individuals, respectively. Although not all cases of NAFLD are MAFLD [93, 111], by adopting the MAFLD terminology, the clinicians can better understand the pathophysiological mechanisms of disease. In terms of risk assessment, although evidence is limited, MAFLD (but not pure NAFLD) can be directly related with all-cause mortality [112, 113]. NAFLD has been linked with the development of cardiovascular diseases [114], although this association seems mainly linked with the metabolic components. In fact, NAFLD subjects not classifiable as MAFLD are at lower risk [21, 115]. Therapeutic approaches will improve since MAFLD points to the interaction of several pathophysiological factors and the multidisciplinary collaboration between internists, cardiologists, endocrinologists, nutritionists, hepatologists, and family medicine [10, 11, 83, 91, 94, 115,116,117,118,119,120,121,122]. In addition, the target populations will gain a better comprehension of terminology [88, 123] without feeling stigmatized because of the word “alcoholic” [12, 119, 121, 124].
The change in terminology, along with either exclusion or inclusion criteria for NAFLD and MAFLD, respectively, creates three groups partially overlapping (Fig. 2B):
-
“Pure” NAFLD (non-MAFLD) where metabolic dysfunction is absent, and significant alcohol intake is excluded.
-
“Overlapping” N(M)AFLD where metabolic dysfunction is present and significant alcohol intake is excluded.
-
“Pure” MAFLD (non-NAFLD) where metabolic dysfunction is present and alcohol consumption can be significant.
Furthermore, in terms of risk assessment related to metabolic phenotypes (all-cause mortality risk, cardiovascular risk, histological progression of liver disease), subjects with overlapping or pure MAFLD represent a heterogeneous group. We speculate that further studies should assess the suitability of a more detailed risk stratification of MAFLD based on specific metabolic phenotypes (Table 2). Recent evidence in a large Korean cohort reported a higher cardiovascular disease risk in lean MAFLD or MAFLD associated with diabetes mellitus, than in overweigh MAFLD subjects, irrespective of metabolic abnormalities or comorbidities. In this cohort, the cardiovascular risk was linked with advanced liver fibrosis irrespective of MAFLD subtype [125].
It is evident that further studies are required to better focus on risk-specific sub-profiles (adjusted for confounders) and natural history of MAFLD/NAFLD association between MAFLD and hypertension and diabetes in the last two groups [126]. Despite few studies have dealt with the comparison between NAFLD and MAFLD, we still need caution due to the nature of the studies (retrospective), selection of groups, statistical issues and conclusions of the studies [96, 127].
Fatty liver disease: more acronyms on the way
Notably, a further classification of liver steatosis is being proposed under the general umbrella of fatty liver disease (FLD). Sub-classifications include almost all possible combinations of genetic, lipodystrophy, metabolic, alcoholic, combined, and yet-to-be-defined causes. This schematic classification will stimulate a further discussion with the aim to improve both comprehension and diagnostic/therapeutic approaches for FLD populations (Table 3) (Fig. 3) [84]. The discussion has gone further with a novel taxonomic classification of NAFLD based on hepatic, pathogenic and systemic features of disease in the individual patient [86, 128]. The liver-determinant-extrahepatic (LDE) system applies to NAFLD and MAFLD and combines information on liver status independently of histology (L), determinants which include sex and reproductive status, genetic, and endocrine assessment (D), and extrahepatic manifestations at a metabolic, cardiovascular, and tumor level (E).
Venn diagrams summarizing the current debate about the nomenclature of NAFLD in relation to other causes of fatty liver disease (FLD). The paradigm shifts from a diagnosis of exclusion («non-alcoholic») to active pathophysiologically established diagnoses involving alcohol abuse, metabolic, genetic, lipodystrophic, combined and yet-to-be-defined causes. AAFLD alcoholic-associated fatty liver disease, CAFLD combined causes of FLD, GAFLD genetics-associated fatty liver disease, LAFLD lipodystrophy-associated fatty liver disease, MAFLD metabolic-associated fatty liver disease, NAFLD non-alcoholic fatty liver disease, XAFLD yet-to-be-defined subgroups
As for MAFLD, however, any sub-classification linked to liver steatosis needs validation studies with terminology to be agreed upon [84]. While searching for better classification of liver steatosis, both researchers, clinicians, scientific societies, patients’ associations, and other stakeholders must be aware that change in terminology requires a better understanding of the molecular basis of the disease entity. Benefits of patients must be balanced along with novel risk stratification and characteristics of disease [87].
Conclusions and future perspectives
The rapid epidemiological increment and the global diffusion of NAFLD is a matter of major concern in terms of healthcare and social burden of disease. Both etiology and pathogenesis of NAFLD are largely unknown, and a multidisciplinary approach is required to handle a frequent liver disease still missing a definitive therapy, beside lifestyle, and maintenance or achievement of ideal body weight. The ongoing discussion urges to revise the terminology [11, 12] since the acronym NAFLD has several limitations: it suggests ignorance about true etiology, it remains an exclusion criterium, it can be ambiguous and misleading, and appears to stigmatize the affected individuals because of the word “alcoholic”. Since NAFLD is very often and closely associated with metabolic dysfunctions, the current view is to shift the acronym NAFLD to MAFLD, i.e., metabolic-associated fatty liver disease. MAFLD becomes an “active” diagnosis based on the presence of overweight/obesity or, in the lean subject, on the combination of metabolic dysfunctions which act as high‐risk factors for events. MAFLD is independent of alcohol intake and the co‐existing causes of liver disease. The change in terminology is likely to improve the classification of affected individuals, the disease awareness, the comprehension of terminology and pathophysiological aspects involved, the choice of more personalized therapeutic approaches, while avoiding the intrinsic stigmatization due to the term “non-alcoholic” (Table 4).
Even more recently, other sub-classification have been proposed, e.g., the LDE terminology [128], and a detailed nomenclature has been proposed to concentrate the heterogeneous causes of fatty liver disease under one umbrella [84] (Table 3). We must take note of such shifts of paradigm and contribute to advance the discussion further. Several partners must agree upon a novel terminology, including clinicians, researchers, pharmaceutical industries, patients and their associations, and scientific societies. In this multidisciplinary field, we need motivated and dedicated researchers with holistic views, to bring tangible pathophysiological, diagnostic, and therapeutic benefits for the populations worldwide suffering from fatty liver disease and related burden of disease. Along with this shift of paradigm, i.e., NAFLD vs. MAFLD, the role of internal medicine and internists is undoubtedly gaining even more trust.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Change history
15 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11739-023-03252-5
References
Ludwig J, Viggiano TR, McGill DB, Oh BJ (1980) Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clinic proceedings. Mayo Clinic 55(7):434–438
Schaffner F, Thaler H (1986) Nonalcoholic fatty liver disease. Prog Liver Dis 8:283–298
Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A (2020) COVID-19 and non-alcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Invest 50(10):e13338. https://doi.org/10.1111/eci.13338
Molina-Molina E, Krawczyk M, Stachowska E, Lammert F, Portincasa P (2019) Non-alcoholic fatty liver disease in non-obese individuals: prevalence, pathogenesis and treatment. Clin Res Hepatol Gastroenterol 43(6):638–645. https://doi.org/10.1016/j.clinre.2019.04.005
Krawczyk M, Portincasa P, Lammert F (2013) PNPLA3-associated steatohepatitis: toward a gene-based classification of fatty liver disease. Semin Liver Dis 33(4):369–379. https://doi.org/10.1055/s-0033-1358525
Krawczyk M, Bonfrate L, Portincasa P (2010) Nonalcoholic fatty liver disease. Best practice & research. Clin Gastroenterol 24(5):695–708. https://doi.org/10.1016/j.bpg.2010.08.005
Targher G, Tilg H, Byrne CD (2021) Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol 6(7):578–588. https://doi.org/10.1016/S2468-1253(21)00020-0
European Association for the Study of the L, European Association for the Study of D, and European Association for the Study of O (2016) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64(6):1388–1402. https://doi.org/10.1016/j.jhep.2015.11.004
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ (2018) The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1):328–357. https://doi.org/10.1002/hep.29367
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wong VW-S, Dufour J-F, Schattenberg JM (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73(1):202–209
Eslam M, Sanyal AJ, George J, International Consensus P (2020) MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158(7):1999-2014 e1991. https://doi.org/10.1053/j.gastro.2019.11.312
Mendez-Sanchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, Sarin SK, Fabrellas N, Zelber-Sagi S, Fan JG, Shiha G, Targher G, Zheng MH, Chan WK, Vinker S, Kawaguchi T, Castera L, Yilmaz Y, Korenjak M, Spearman CW, Ungan M, Palmer M, El-Shabrawi M, Gruss HJ, Dufour JF, Dhawan A, Wedemeyer H, George J, Valenti L, Fouad Y, Romero-Gomez M, Eslam M, Global multi-stakeholder consensus on the redefinition of fatty liver d (2022) Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol 7(5):388–390. https://doi.org/10.1016/S2468-1253(22)00062-0
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB (2019) Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69(6):2672–2682. https://doi.org/10.1002/hep.30251
Cholongitas E, Pavlopoulou I, Papatheodoridi M, Markakis GE, Bouras E, Haidich AB, Papatheodoridis G (2021) Epidemiology of nonalcoholic fatty liver disease in Europe: a systematic review and meta-analysis. Ann Gastroenterol 34(3):404–414. https://doi.org/10.20524/aog.2021.0604
Rojano A, Sena E, Manzano-Nunez R, Pericas JM, Ciudin A (2022) NAFLD as the metabolic hallmark of obesity. Intern Emerg Med. https://doi.org/10.1007/s11739-022-03139-x
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ (2018) Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67(1):123–133. https://doi.org/10.1002/hep.29466
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H (2018) Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol 69(4):896–904. https://doi.org/10.1016/j.jhep.2018.05.036
Loomba R, Adams LA (2019) The 20% rule of NASH progression: the natural history of advanced fibrosis and cirrhosis caused by NASH. Hepatology 70(6):1885–1888. https://doi.org/10.1002/hep.30946
Wong T, Dang K, Ladhani S, Singal AK, Wong RJ (2019) Prevalence of alcoholic fatty liver disease among adults in the United States, 2001–2016. JAMA 321(17):1723–1725. https://doi.org/10.1001/jama.2019.2276
Powell EE, Wong VW, Rinella M (2021) Non-alcoholic fatty liver disease. Lancet 397(10290):2212–2224. https://doi.org/10.1016/S0140-6736(20)32511-3
Lee H, Lee YH, Kim SU, Kim HC (2021) Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol 19(10):2138-2147 e2110. https://doi.org/10.1016/j.cgh.2020.12.022
Li AA, Kim D, Ahmed A (2020) Association of sarcopenia and NAFLD: an overview. Clin Liver Dis (Hoboken) 16(2):73–76. https://doi.org/10.1002/cld.900
Polyzos SA, and Mantzoros CS (2020). Making progress in nonalcoholic fatty liver disease (NAFLD) as we are transitioning from the era of NAFLD to dys-metabolism associated fatty liver disease (DAFLD). Metab-Clin Exp 111
Hagstrom H, Simon TG, Roelstraete B, Stephansson O, Soderling J, Ludvigsson JF (2021) Maternal obesity increases the risk and severity of NAFLD in offspring. J Hepatol 75(5):1042–1048. https://doi.org/10.1016/j.jhep.2021.06.045
Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, Young B, Krebs N, Lemas DJ, Johnson LK, Weir T, Lenz LL, Frank DN, Hernandez TL, Kuhn KA, D’Alessandro A, Barbour LA, El Kasmi KC, Friedman JE (2018) The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun 9(1):4462. https://doi.org/10.1038/s41467-018-06929-0
Arrese M, Arab JP, Barrera F, Kaufmann B, Valenti L, Feldstein AE (2021) Insights into nonalcoholic fatty-liver disease heterogeneity. Semin Liver Dis 41(4):421–434. https://doi.org/10.1055/s-0041-1730927
Semmler G, Datz C, Reiberger T, Trauner M (2021) Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int 41(10):2249–2268. https://doi.org/10.1111/liv.15024
Angelidi AM, Papadaki A, Nolen-Doerr E, Boutari C, Mantzoros CS (2022) The effect of dietary patterns on non-alcoholic fatty liver disease diagnosed by biopsy or magnetic resonance in adults: a systematic review of randomised controlled trials. Metab Clin Exp 129:155136. https://doi.org/10.1016/j.metabol.2022.155136
Li FR, Liao J, Zhu B, Li X, Cheng Z, Jin C, Mo C, Wu X, Li Q, Liang F (2022) Long-term exposure to air pollution and incident non-alcoholic fatty liver disease and cirrhosis: a cohort study. Liver Int. https://doi.org/10.1111/liv.15416
Guo B, Guo Y, Nima Q, Feng Y, Wang Z, Lu R, Baimayangji MY, Zhou J, Xu H, Chen L, Chen G, Li S, Tong H, Ding X, Zhao X, Cohort CM-E, collaborative g (2022) Exposure to air pollution is associated with an increased risk of metabolic dysfunction-associated fatty liver disease. J Hepatol 76(3):518–525. https://doi.org/10.1016/j.jhep.2021.10.016
Di Ciaula A, Portincasa P (2019) Diet and contaminants: driving the rise to obesity epidemics? Curr Med Chem 26(19):3471–3482. https://doi.org/10.2174/0929867324666170518095736
Alferink LJM, Radjabzadeh D, Erler NS, Vojinovic D, Medina-Gomez C, Uitterlinden AG, de Knegt RJ, Amin N, Ikram MA, Janssen HLA, Kiefte-de Jong JC, Metselaar HJ, van Duijn CM, Kraaij R, Darwish Murad S (2021) Microbiomics, metabolomics, predicted metagenomics, and hepatic steatosis in a population-based study of 1,355 adults. Hepatology 73(3):968–982. https://doi.org/10.1002/hep.31417
Smyk W, Janik MK, Portincasa P, Milkiewicz P, Lammert F, Krawczyk M (2020) COVID-19: focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Investig 50(9):e13276
Di Ciaula A, Bonfrate L, Portincasa P (2022) The role of microbiota in nonalcoholic fatty liver disease. Eur J Clin Invest 52(7):e13768. https://doi.org/10.1111/eci.13768
Vecchie A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, Fruhbeck G, Montecucco F (2018) Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med 48:6–17. https://doi.org/10.1016/j.ejim.2017.10.020
Francque S, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, Van Gaal L, Michielsen P (2010) Noncirrhotic human nonalcoholic fatty liver disease induces portal hypertension in relation to the histological degree of steatosis. Eur J Gastroenterol Hepatol 22(12):1449–1457. https://doi.org/10.1097/MEG.0b013e32833f14a1
Di Ciaula A, Carbone F, Shanmugham H, Molina-Molina E, Bonfrate L, Ministrini S, Montecucco F, Portincasa P (2021) Adiponectin involved in portal flow hepatic extraction of 13C-methacetin in obesity and non-alcoholic fatty liver. Eur J Intern Med 89:56–64. https://doi.org/10.1016/j.ejim.2021.03.036
Pasarin M, La Mura V, Gracia-Sancho J, Garcia-Caldero H, Rodriguez-Vilarrupla A, Garcia-Pagan JC, Bosch J, Abraldes JG (2012) Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS ONE 7(4):e32785. https://doi.org/10.1371/journal.pone.0032785
Ryou M, Stylopoulos N, Baffy G (2020) Nonalcoholic fatty liver disease and portal hypertension. Explor Med 1:149–169. https://doi.org/10.37349/emed.2020.00011
Di Ciaula A, Passarella S, Shanmugam H, Noviello M, Bonfrate L, Wang DQ-H, Portincasa P (2021) Nonalcoholic fatty liver disease (NAFLD). Mitochondria as players and targets of therapies? Int J Mol Sci 22(10):5375
Rinella ME, Sanyal AJ (2016) Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol 13(4):196–205. https://doi.org/10.1038/nrgastro.2016.3
Portincasa P, Wang DQH (2017) Nonalcoholic fatty liver and gallstone disease. In: Wang DQH, Portincasa P (eds) Gallstones. Recent advances in epidemiology, pathogenesis, diagnosis and management. Nova Science Publisher Inc, USA, pp 387–414
Grattagliano I, De Bari O, Di Palo D, Montecucco F, Carbone F, Oliveira P, Wang DQH, Portincasa P (2018) Mitochondria in liver diseases. In: Oliveira P (ed) Mitochondrial biology and experimental therapeutics. Springer Nature, Berlin, pp 91–126
Chen Z, Yu Y, Cai J, Li H (2019) Emerging molecular targets for treatment of nonalcoholic fatty liver disease. Trends Endocrinol Metab: TEM 30(12):903–914. https://doi.org/10.1016/j.tem.2019.08.006
Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R (2017) Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 65(5):1557–1565. https://doi.org/10.1002/hep.29085
Di Ciaula A, Baj J, Garruti G, Celano G, De Angelis M, Wang HH, Di Palo DM, Bonfrate L, Wang DQ, Portincasa P (2020) Liver steatosis, gut-liver axis, microbiome and environmental factors. A never-ending bidirectional cross-talk. J Clin Med 9(8):2648. https://doi.org/10.3390/jcm9082648
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ, American Gastroenterological A, American Association for the Study of Liver D, and American College of G (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American gastroenterological association, American association for the study of liver diseases, and American college of gastroenterology. Gastroenterology 142(7):1592–1609. https://doi.org/10.1053/j.gastro.2012.04.001
Blomdahl J, Nasr P, Ekstedt M, Kechagias S (2021) Moderate alcohol consumption is associated with advanced fibrosis in non-alcoholic fatty liver disease and shows a synergistic effect with type 2 diabetes mellitus. Metab Clin Exp 115:154439
Patel PJ, Smith D, Connor JP, Horsfall LU, Hayward KL, Hossain F, Williams S, Johnson T, Stuart KA, Brown NN, Saad N, Clouston AD, Irvine KM, Russell AW, Valery PC, Powell EE (2017) Alcohol consumption in diabetic patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2017:7927685. https://doi.org/10.1155/2017/7927685
Di Ciaula A, Bonfrate L, Krawczyk M, Fruhbeck G, Portincasa P (2022) Synergistic and detrimental effects of alcohol intake on progression of liver steatosis. Int J Mol Sci 23(5):2636. https://doi.org/10.3390/ijms23052636
Li JF, Qu F, Zheng SJ, Wu HL, Liu M, Liu S, Ren Y, Ren F, Chen Y, Duan ZP, Zhang JL (2014) Elevated plasma sphingomyelin (d18:1/22:0) is closely related to hepatic steatosis in patients with chronic hepatitis C virus infection. Eur J Clin Microbiol Infect Dis 33(10):1725–1732. https://doi.org/10.1007/s10096-014-2123-x
Yasui K, Harano Y, Mitsuyoshi H, Tsuji K, Endo M, Nakajima T, Minami M, Itoh Y, Zen Y, Nakanuma Y, Yoshikawa T, Okanoue T (2010) Steatosis and hepatic expression of genes regulating lipid metabolism in Japanese patients infected with hepatitis C virus. J Gastroenterol 45(1):95–104. https://doi.org/10.1007/s00535-009-0133-8
Jian WY, Shu Chen L, Gui Qiang W (2006) Effects of fatty liver and related factors on the efficacy of combination antiviral therapy in patients with chronic hepatitis C. Liver Int 26(2):166–172. https://doi.org/10.1111/j.1478-3231.2005.01219.x
Hwang SJ, Luo JC, Chu CW, Lai CR, Lu CL, Tsay SH, Wu JC, Chang FY, Lee SD (2001) Hepatic steatosis in chronic hepatitis C virus infection: prevalence and clinical correlation. J Gastroenterol Hepatol 16(2):190–195. https://doi.org/10.1046/j.1440-1746.2001.02407.x
Satapathy SK, Kuwajima V, Nadelson J, Atiq O, Sanyal AJ (2015) Drug-induced fatty liver disease: an overview of pathogenesis and management. Ann Hepatol 14(6):789–806. https://doi.org/10.5604/16652681.1171749
Stattermayer AF, Traussnigg S, Dienes HP, Aigner E, Stauber R, Lackner K, Hofer H, Stift J, Wrba F, Stadlmayr A, Datz C, Strasser M, Maieron A, Trauner M, Ferenci P (2015) Hepatic steatosis in Wilson disease–role of copper and PNPLA3 mutations. J Hepatol 63(1):156–163. https://doi.org/10.1016/j.jhep.2015.01.034
Jordan T, Popovic P, Rotovnik Kozjek N (2020) Liver steatosis in adult patients on home parenteral nutrition. Eur J Clin Nutr 74(2):255–260. https://doi.org/10.1038/s41430-019-0455-4
Grattagliano I, Di Ciaula A, Baj J, Molina-Molina E, Shanmugam H, Garruti G, Wang DQ, Portincasa P (2021) Protocols for mitochondria as the target of pharmacological therapy in the context of nonalcoholic fatty liver disease (NAFLD). Methods Mol Biol 2310:201–246. https://doi.org/10.1007/978-1-0716-1433-4_12
Angulo P (2007) Obesity and nonalcoholic fatty liver disease. Nutr Rev 65(6 Pt 2):S57-63. https://doi.org/10.1111/j.1753-4887.2007.tb00329.x
Liu CJ (2012) Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol 27(10):1555–1560. https://doi.org/10.1111/j.1440-1746.2012.07222.x
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15(1):11–20. https://doi.org/10.1038/nrgastro.2017.109
Yarnoz-Esquiroz P, Olazaran L, Aguas-Ayesa M, Perdomo CM, Garcia-Goni M, Silva C, Fernandez-Formoso JA, Escalada J, Montecucco F, Portincasa P, Fruhbeck G (2022) “Obesities”: position statement on a complex disease entity with multifaceted drivers. Eur J Clin Investig 52(7):e13811. https://doi.org/10.1111/eci.13811
Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34(3):274–285. https://doi.org/10.1111/j.1365-2036.2011.04724.x
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140(1):124–131. https://doi.org/10.1053/j.gastro.2010.09.038
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84. https://doi.org/10.1002/hep.28431
Byrne CD, Targher G (2015) NAFLD: a multisystem disease. J Hepatol 62(1 Suppl):S47-64. https://doi.org/10.1016/j.jhep.2014.12.012
Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M (2011) Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 9(6):524–530. https://doi.org/10.1016/j.cgh.2011.03.020
Molina-Molina E, Lunardi Baccetto R, Wang DQ, de Bari O, Krawczyk M, Portincasa P (2018) Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur J Clin Investig 48(8):e12958. https://doi.org/10.1111/eci.12958
Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H (2020) Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology 71(5):1851–1864. https://doi.org/10.1002/hep.31150
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL, GBD 215 Obesity Collaborators (2017) Health effects of overweight and obesity in 195 Countries over 25 years. New Engl J Med 377(1):13–27. https://doi.org/10.1056/NEJMoa1614362
Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F (2019) The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 71(4):793–801. https://doi.org/10.1016/j.jhep.2019.06.021
Brouwers M, Simons N, Stehouwer CDA, Isaacs A (2020) Non-alcoholic fatty liver disease and cardiovascular disease: assessing the evidence for causality. Diabetologia 63(2):253–260. https://doi.org/10.1007/s00125-019-05024-3
Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y (2020) Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int 40(7):1594–1600. https://doi.org/10.1111/liv.14461
Anstee QM, Mantovani A, Tilg H, Targher G (2018) Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 15(7):425–439. https://doi.org/10.1038/s41575-018-0010-0
Tsochatzis EA, Newsome PN (2018) Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol 3(7):509–517. https://doi.org/10.1016/s2468-1253(18)30077-3
Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R (2016) The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64(5):1577–1586. https://doi.org/10.1002/hep.28785
Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM (2020) Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 72(5):1605–1616. https://doi.org/10.1002/hep.31173
Kim D, Kim WR (2017) Nonobese fatty liver disease. Clin Gastroenterol Hepatol 15(4):474–485. https://doi.org/10.1016/j.cgh.2016.08.028
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S (2020) Nonalcoholic steatohepatitis: a review. JAMA 323(12):1175–1183. https://doi.org/10.1001/jama.2020.2298
Stepanova M, Younossi ZM (2012) Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 10(6):646–650. https://doi.org/10.1016/j.cgh.2011.12.039
Catalan V, Aviles-Olmos I, Rodriguez A, Becerril S, Fernandez-Formoso JA, Kiortsis D, Portincasa P, Gomez-Ambrosi J, Fruhbeck G (2022) Time to consider the “exposome hypothesis” in the development of the obesity pandemic. Nutrients. https://doi.org/10.3390/nu14081597
Di Ciaula A, Calamita G, Shanmugam H, Khalil M, Bonfrate L, Wang DQ, Baffy G, Portincasa P (2021) Mitochondria matter: systemic aspects of nonalcoholic fatty liver disease (NAFLD) and diagnostic assessment of liver function by stable isotope dynamic breath tests. Int J Mol Sci 22(14):7702. https://doi.org/10.3390/ijms22147702
Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Hogstrom S, Richardson B, Munoz C, Sigurethardottir S, Coulibaly A, Milan M, Bautista F, Leung NWY, Mooney V, Obekpa S, Bech E, Polavarapu N, Hamed AE, Radiani T, Purwanto E, Bright B, Ali M, Dovia CK, McColaugh L, Koulla Y, Dufour JF, Soliman R, Eslam M (2021) Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol 6(1):73–79. https://doi.org/10.1016/S2468-1253(20)30294-6
Valenzuela-Vallejo L, Mantzoros CS (2022) Time to transition from a negative nomenclature describing what NAFLD is not, to a novel, pathophysiology-based, umbrella classification of fatty liver disease (FLD). Metab Clin Exp 134:155246. https://doi.org/10.1016/j.metabol.2022.155246
Alqahtani SA, Paik JM, Biswas R, Arshad T, Henry L, Younossi ZM (2021) Poor awareness of liver disease among adults with NAFLD in the United States. Hepatol Commun 5(11):1833–1847
Lonardo A, Singal AK, Osna N, Kharbanda KK (2022) Effect of cofactors on NAFLD/NASH and MAFLD. A paradigm illustrating the pathomechanics of organ dysfunction. Metab Target Organ Damage. https://doi.org/10.2057/mtod.2022.14
Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, Cohen DE, Loomba R (2021) From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology 73(3):1194–1198. https://doi.org/10.1002/hep.31420
Mendez-Sanchez N, Diaz-Orozco L, Cordova-Gallardo J (2021) Redefinition of fatty liver disease from NAFLD to MAFLD raised disease awareness: Mexican experience. J Hepatol 75(1):221–222. https://doi.org/10.1016/j.jhep.2021.04.021
Nan Y, An J, Bao J, Chen H, Chen Y, Ding H, Dou X, Duan Z, Fan J, Gao Y, Han T, Han Y, Hu P, Huang Y, Huang Y, Jia J, Jiang J, Jiang Y, Li J, Li J, Li R, Li S, Li W, Li Y, Lin S, Liu J, Liu S, Lu L, Lu Q, Luo X, Ma X, Rao H, Ren H, Ren W, Shang J, Shi L, Su M, Wang B, Wang R, Wei L, Wen Z, Wu B, Wu J, Xin S, Xing H, Xu J, Yan M, Yang J, Yang J, Yang L, Yang Y, Yu Y, Zhang L, Zhang L, Zhang X, Zhang Y, Zhang Y, Zhao J, Zhao S, Zheng H, Zhou Y, Zhou Y, Zhuang H, Zuo W, Xu X, Qiao L (2021) The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol 75(2):454–461. https://doi.org/10.1016/j.jhep.2021.05.003
Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J (2020) The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 14(6):889–919. https://doi.org/10.1007/s12072-020-10094-2
Shiha G, Alswat K, Al Khatry M, Sharara AI, Ormeci N, Waked I, Benazzouz M, Al-Ali F, Hamed AE, Hamoudi W, Attia D, Derbala M, Sharaf-Eldin M, Al-Busafi SA, Zaky S, Bamakhrama K, Ibrahim N, Ajlouni Y, Sabbah M, Salama M, Anushiravani A, Afredj N, Barakat S, Hashim A, Fouad Y, Soliman R (2021) Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and north Africa. Lancet Gastroenterol Hepatol 6(1):57–64. https://doi.org/10.1016/S2468-1253(20)30213-2
Tilg H, Effenberger M (2020) From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol 17(7):387–388. https://doi.org/10.1038/s41575-020-0316-6
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y (2020) Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int 40(9):2082–2089. https://doi.org/10.1111/liv.14548
Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T (2020) MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int 40(12):3018–3030. https://doi.org/10.1111/liv.14675
The Lancet G Hepatology (2020) Redefining non-alcoholic fatty liver disease: what’s in a name? Lancet Gastroenterol Hepatol 5(5):419. https://doi.org/10.1016/s2468-1253(20)30091-1
Kawaguchi T, Tsutsumi T, Nakano D, Torimura T (2022) MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res 52(5):422–432. https://doi.org/10.1111/hepr.13706
Park H, Yoon EL, Cho S, Jun DW, Nah E-H (2022) Diabetes is the strongest risk factor of hepatic fibrosis in lean patients with non-alcoholic fatty liver disease. Gut 71(5):1035–1036
Kawaguchi T, Torimura T (2020) Is metabolic syndrome responsible for the progression from NAFLD to NASH in non-obese patients? Springer
Ochiai Y, Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Muraishi N, Kajiwara A, Iritani S, Fujiyama S, Hosaka T, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ikeda K, Arase Y, Hashimoto M, Kumada H (2021) Effects of alcohol consumption on multiple hepatocarcinogenesis in patients with fatty liver disease. Hepatol Res 51(1):62–68. https://doi.org/10.1111/hepr.13572
Xu L, Xie J, Chen S, Chen Y, Yang H, Miao M, Zhu Z, Li Y, Yu C, Xu C (2020) Light-to-moderate alcohol consumption is associated with increased risk of type 2 diabetes in individuals with nonalcoholic fatty liver disease: a nine-year cohort study. Off J Am College Gastroenterol ACG 115(6):876–884
Hajifathalian K, Torabi Sagvand B, McCullough AJ (2019) Effect of alcohol consumption on survival in nonalcoholic fatty liver disease: a national prospective cohort study. Hepatology 70(2):511–521. https://doi.org/10.1002/hep.30226
Chang Y, Cho YK, Kim Y, Sung E, Ahn J, Jung HS, Yun KE, Shin H, Ryu S (2019) Nonheavy drinking and worsening of noninvasive fibrosis markers in nonalcoholic fatty liver disease: a cohort study. Hepatology 69(1):64–75. https://doi.org/10.1002/hep.30170
Fuster D, Samet JH (2018) Alcohol use in patients with chronic liver disease. New Engl J Med 379(13):1251–1261
Choi HS, Brouwer WP, Zanjir WM, de Man RA, Feld JJ, Hansen BE, Janssen HL, Patel K (2020) Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology 71(2):539–548
Tada T, Nishimura T, Matono T, Yoshida M, Yuri M, Fujiwara A, Yuri Y, Takashima T, Aizawa N, Ikeda N, Enomoto H, Kumada T, Iijima H (2021) Association of liver stiffness and steatosis with hepatocellular carcinoma development in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. Hepatol Res 51(8):860–869. https://doi.org/10.1111/hepr.13677
Guerreiro GTS, Longo L, Fonseca MA, de Souza VEG, Alvares-da-Silva MR (2021) Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD? Hepatol Int 15(2):380–391
Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S, Aoyama T, Tanaka E (2011) Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol 46(6):758–768. https://doi.org/10.1007/s00535-011-0370-5
Pavlik L, Regev A, Ardayfio PA, Chalasani NP (2019) Drug-induced steatosis and steatohepatitis: the search for novel serum biomarkers among potential biomarkers for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Drug Saf 42(6):701–711
Sasamori Y, Tanaka A, Ayabe T (2020) Liver disease in pregnancy. Hepatol Res 50(9):1015–1023. https://doi.org/10.1111/hepr.13540
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C (2006) The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6:33. https://doi.org/10.1186/1471-230X-6-33
Bianco C, Romeo S, Petta S, Long MT, Valenti L (2020) MAFLD vs NAFLD: let the contest begin! Liver Int 40(9):2079–2081. https://doi.org/10.1111/liv.14620
Xie J, Lu L, Chen Y, Xu L, Xu C (2022) A simpler definition of MAFLD better predicts long-term all-cause mortality in American adults. J Hepatol 77(3):877–879. https://doi.org/10.1016/j.jhep.2022.01.015
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A (2021) Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol 75(6):1284–1291. https://doi.org/10.1016/j.jhep.2021.07.035
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C (2016) Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 65(3):589–600. https://doi.org/10.1016/j.jhep.2016.05.013
Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, Koseki M, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T (2021) MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res 51(11):1115–1128. https://doi.org/10.1111/hepr.13685
Eslam M, Ahmed A, Despres JP, Jha V, Halford JCG, Wei Chieh JT, Harris DCH, Nangaku M, Colagiuri S, Targher G, Joshi S, Byrne CD, Khunti K, Nguyen MH, Gish RG, George J (2021) Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol 6(9):743–753. https://doi.org/10.1016/S2468-1253(21)00132-1
Ayada I, van Kleef LA, Alferink LJM, Li P, de Knegt RJ, Pan Q (2022) Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta-analysis: focusing on the non-overlap groups. Liver Int 42(2):277–287. https://doi.org/10.1111/liv.15139
van Kleef LA, Ayada I, Alferink LJM, Pan Q, de Knegt RJ (2022) Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: the rotterdam study. Hepatology 75(2):419–429. https://doi.org/10.1002/hep.32131
Mendez-Sanchez N, Arrese M, Gadano A, Oliveira CP, Fassio E, Arab JP, Chavez-Tapia NC, Dirchwolf M, Torre A, Ridruejo E, Pinchemel-Cotrim H, Castellanos Fernandez MI, Uribe M, Girala M, Diaz-Ferrer J, Restrepo JC, Padilla-Machaca M, Dagher L, Gatica M, Olaechea B, Pessoa MG, Silva M (2021) The Latin American association for the study of the liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol 6(1):65–72. https://doi.org/10.1016/S2468-1253(20)30340-X
Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, Socha P, Marcus C, Lee WS, Kelly D, Porta G, El-Guindi MA, Alisi A, Mann JP, Mouane N, Baur LA, Dhawan A, George J (2021) Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol 6(10):864–873. https://doi.org/10.1016/S2468-1253(21)00183-7
Shiha G, Korenjak M, Casanovas T, Mooney V, Sigurethardottir S, Koulla Y, Soliman R (2022) MAFLD 2022: an ELPA/ALPA/EASO-ECPO joint statement on disease stigma. J Hepatol. https://doi.org/10.1016/j.jhep.2022.08.027
Alharthi J, Gastaldelli A, Cua IH, Ghazinian H, Eslam M (2022) Metabolic dysfunction-associated fatty liver disease: a year in review. Curr Opin Gastroenterol 38(3):251–260. https://doi.org/10.1097/MOG.0000000000000823
Fouad Y, Gomaa A, Semida N, Ghany WA, Attia D (2021) Change from NAFLD to MAFLD increases the awareness of fatty liver disease in primary care physicians and specialists. J Hepatol 74(5):1254–1256. https://doi.org/10.1016/j.jhep.2020.12.035
Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, Pryke R, Hutchinson SJ, Sangro B, Martin NK, Cecchini M, Dirac MA, Belloni A, Serra-Burriel M, Ponsioen CY, Sheena B, Lerouge A, Devaux M, Scott N, Hellard M, Verkade HJ, Sturm E, Marchesini G, Yki-Jarvinen H, Byrne CD, Targher G, Tur-Sinai A, Barrett D, Ninburg M, Reic T, Taylor A, Rhodes T, Treloar C, Petersen C, Schramm C, Flisiak R, Simonova MY, Pares A, Johnson P, Cucchetti A, Graupera I, Lionis C, Pose E, Fabrellas N, Ma AT, Mendive JM, Mazzaferro V, Rutter H, Cortez-Pinto H, Kelly D, Burton R, Lazarus JV, Gines P, Buti M, Newsome PN, Burra P, Manns MP (2022) The EASL-lancet liver commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 399(10319):61–116. https://doi.org/10.1016/S0140-6736(21)01701-3
Lee H, Lim TS, Kim SU, Kim HC (2022) Long-term cardiovascular outcomes differ across metabolic dysfunction-associated fatty liver disease subtypes among middle-aged population. Hepatol Int 16(6):1308–1317. https://doi.org/10.1007/s12072-022-10407-7
Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, Tan DJH, Lim WH, Lin SY, Huang D, Chan M, Khoo CM, Chew NWS, Kaewdech A, Chamroonkul N, Dan YY, Noureddin M, Muthiah M, Eslam M, Ng CH (2022) Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10 739 607 individuals. J Clin EndocrinolMetab 107(9):2691–2700. https://doi.org/10.1210/clinem/dgac321
Mantovani A (2021) MAFLD vs NAFLD: where are we? Dig Liver Dis 53(10):1368–1372
Lonardo A, Ballestri S (2020) Perspectives of nonalcoholic fatty liver disease research: a personal point of view. Explor Med 1(3):85–107. https://doi.org/10.37349/emed.2020.00007
Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS (2010) Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 42(7):503–508. https://doi.org/10.1016/j.dld.2009.08.002
Lee DH, Cho EJ, Bae JS, Lee JY, Yu SJ, Kim H, Lee KB, Han JK, Choi BI (2021) Accuracy of two-dimensional shear wave elastography and attenuation imaging for evaluation of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 19(4):797-805 e797. https://doi.org/10.1016/j.cgh.2020.05.034
Ferraioli G (2021) Quantitative assessment of liver steatosis using ultrasound controlled attenuation parameter (Echosens). J Med Ultrason 48(4):489–495. https://doi.org/10.1007/s10396-021-01106-1
Younossi ZM, Page S, Rafiq N, Birerdinc A, Stepanova M, Hossain N, Afendy A, Younoszai Z, Goodman Z, Baranova A (2011) A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obesity Surg 21(4):431–439. https://doi.org/10.1007/s11695-010-0204-1
Castera L, Friedrich-Rust M, Loomba R (2019) Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156(5):1264-1281 e1264. https://doi.org/10.1053/j.gastro.2018.12.036
Lonardo A (2021) Back to the future: from the history of NAFLD to MAFLD to heterogeneity of disease. Clin Transl Discov 1(1):e9. https://doi.org/10.1002/ctd2.9
Polyzos SA, Kountouras J, Zavos C (2009) Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med 9(3):299–314. https://doi.org/10.2174/156652409787847191
Passarella S, Schurr A, Portincasa P (2021) Mitochondrial transport in glycolysis and gluconeogenesis: achievements and perspectives. Int J Mol Sci. https://doi.org/10.3390/ijms222312620
Schmitz SM, Schooren L, Kroh A, Koch A, Stier C, Neumann UP, Ulmer TF, Alizai PH (2021) Association of body composition and sarcopenia with NASH in obese patients. J Clin Med 10(15):3445. https://doi.org/10.3390/jcm10153445
Lee JH, Jun HS (2019) Role of myokines in regulating skeletal muscle mass and function. Front Physiol 10:42. https://doi.org/10.3389/fphys.2019.00042
Perakakis N, Mougios V, Fatouros I, Siopi A, Draganidis D, Peradze N, Ghaly W, Mantzoros CS (2018) Physiology of activins/follistatins: associations with metabolic and anthropometric variables and response to exercise. J Clin Endocrinol Metab 103(10):3890–3899. https://doi.org/10.1210/jc.2018-01056
Bril F (2021) What the new definition of metabolic dysfunction-associated fatty liver disease (MAFLD) left behind: genetically acquired fatty liver disease (GAFLD). EBioMedicine 72:103584. https://doi.org/10.1016/j.ebiom.2021.103584
Fiorenza CG, Chou SH, Mantzoros CS (2011) Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol 7(3):137–150. https://doi.org/10.1038/nrendo.2010.199
Toshikuni N, Tsutsumi M, Arisawa T (2014) Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J Gastroenterol: WJG 20(26):8393–8406. https://doi.org/10.3748/wjg.v20.i26.8393
Chalasani S, Mathur K, Shammas N, Orman E, Vuppalanchi R, Lammert C (2020) Hepatic steatosis is highly prevalent but is not correlated with stiffness in autoimmune hepatitis. Medicine (Baltimore) 99(42):e22805. https://doi.org/10.1097/MD.0000000000022805
Haga Y, Kanda T, Sasaki R, Nakamura M, Nakamoto S, Yokosuka O (2015) Nonalcoholic fatty liver disease and hepatic cirrhosis: comparison with viral hepatitis-associated steatosis. World J Gastroenterol: WJG 21(46):12989–12995. https://doi.org/10.3748/wjg.v21.i46.12989
Krause C, Grohs M, El Gammal AT, Wolter S, Lehnert H, Mann O, Mittag J, Kirchner H (2018) Reduced expression of thyroid hormone receptor beta in human nonalcoholic steatohepatitis. Endocr Connect 7(12):1448–1456. https://doi.org/10.1530/EC-18-0499
Khalil M, Shanmugam H, Abdallah H, John Britto JS, Galerati I, Gomez-Ambrosi J, Fruhbeck G, Portincasa P (2022) The potential of the mediterranean diet to improve mitochondrial function in experimental models of obesity and metabolic syndrome. Nutrients 14(15):3112. https://doi.org/10.3390/nu14153112
Longo M, Meroni M, Paolini E, Macchi C, Dongiovanni P (2021) Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): new perspectives for a fairy-tale ending? Metab Clin Exp 117:154708. https://doi.org/10.1016/j.metabol.2021.154708
Acknowledgements
The author is indebted to Leonilde Bonfrate, Giuseppe Calamita, Agostino Di Ciaula, Domenica Di Palo, Dan Dumitrascu, Karel van Erpecum, Mohamad Khalil, Marcin Krawczyk, Paulo Oliveira, Harshita Shanmugam, and David Q.-H. Wang for helpful scientific discussion and longstanding collaborations.
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement. This paper has been partly supported by funding from the European Union’s Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie Grant Agreement No. 722619 (FOIE GRAS), Grant Agreement No. 734719 (mtFOIE GRAS), Grant Regione Puglia, CUP H99C20000340002 (Fever Apulia), and Grant EUROSEEDS Uniba—S56—By-products Sustainable Recovery 4 Health (BSR-4H): University of Bari Aldo Moro, 2022.
Author information
Authors and Affiliations
Contributions
PP: conceptualization, data curation, funding acquisition, investigation, methodology, software, and writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest.
Human and animal rights statement and Informed consent
The article is a review analysis. Human Participants and/or Animals have not been involved in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the word painless was changed as painful under the section NAFLD and metabolic dysfunctions. also, a grammatical change in text under Conclusions and future perspectives section has been amended.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Portincasa, P. NAFLD, MAFLD, and beyond: one or several acronyms for better comprehension and patient care. Intern Emerg Med 18, 993–1006 (2023). https://doi.org/10.1007/s11739-023-03203-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03203-0