Abstract
We studied the predictive value of the PaO2/FiO2 ratio for classifying COVID-19-positive patients who will develop severe clinical outcomes. One hundred fifty patients were recruited and categorized into two distinct populations (“A” and “B”), according to the indications given by the World Health Organization. Patients belonging the population “A” presented with mild disease not requiring oxygen support, whereas population “B” presented with a severe disease needing oxygen support. The AUC curve of PaO2/FiO2 in the discovery cohort was 0.838 (95% CI 0.771–0.908). The optimal cut-off value for distinguishing population “A” from the “B” one, calculated by Youden’s index, with sensitivity of 71.79% and specificity 85.25%, LR+4.866, LR−0.339, was < 274 mmHg. The AUC in the validation cohort of 170 patients overlapped the previous one, i.e., 0.826 (95% CI 0.760–0.891). PaO2/FiO2 ratio < 274 mmHg was a good predictive index test to forecast the development of a severe respiratory failure in SARS-CoV-2-infected patients. Moreover, our work highlights that PaO2/FiO2 ratio, compared to inflammatory scores (hs-CRP, NLR, PLR and LDH) indicated to be useful in clinical managements, results to be the most reliable parameter to identify patients who require closer respiratory monitoring and more aggressive supportive therapies. Clinical trial registration: Prognostic Score in COVID-19, prot. NCT04780373 https://clinicaltrials.gov/ct2/show/NCT04780373 (retrospectively registered).
Similar content being viewed by others
Introduction
The natural history of SARS-CoV-2 infection is extremely variable, ranging from asymptomatic or mild infection to a severe acute distress respiratory syndrome (ARDS), characterized by a typical hyperinflammatory response associated with a microangiopathy and a widespread thrombosis [1,2,3,4].
In the face of the rapidly spreading disease and the large number of infected people, there is an urgent need to cluster patients in risk categories by identifying reliable parameters related to COVID-19 disease progression to stratify high-risk patients, who will suffer rapid disease progression to severe complications and death.
The clinical respiratory symptoms of SARS-CoV-2 infection frequently do not correspond to the severity of lung damage; therefore, to correctly stratify COVID-19 patients, it is important to evaluate the acute lung injury based on well-accepted test for acute lung injury and ARDS, such as the partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2) [5,6,7,8].
Several inflammatory biomarker abnormalities have been identified to stratify COVID-19 patients who will develop a severe disease [9,10,11]. High-sensitivity C-reactive protein (Hs-CRP) as well as neutrophil (NEU)-to-lymphocyte (LYM) ratio (NLR) and platelet (PLT)-to-lymphocyte (LYM) ratio (PLR) have been investigated in SARS-CoV-2 infection and have been indicated to be useful in clinical managements of infected patients [12, 13]. Moreover, lactate dehydrogenase (LDH), an intracellular enzyme that well correlates with lung damage and inflammation, has been associated with worse outcomes in patients with viral infections and has been identified as an independent indicator for predicting severity and mortality in patients with COVID-19 [14, 15].
The aim of our study was to assess the PaO2/FiO2 ratio as a reliable prognostic biomarker to forecast the progression of COVID-19 patients towards the most severe form, according to WHO criteria, comparing its predictive value with other canonical inflammatory biomarkers.
Materials and methods
Study design
We conducted a cross-sectional monocentric study, adhering to the principles of the Declaration of Helsinki. The study was approved by the institutional ethic committee (License Prot. n. 71726) and informed consent was obtained from participating patients at the San Salvatore Hospital, L’Aquila, Italy.
Discovery cohort
Inclusion criteria
During the first and second pandemic “wave” of SARS-CoV-2 epidemic, from April 15, 2020, to November 31, 2020, 153 patients were recruited at the Department of Infectious Disease and at the Department of Pneumology of the San Salvatore Hospital, L’Aquila, Italy. All patients showed positivity to RT-PCR assay from nasopharyngeal swab sample and clear futures to chest CT. At the arrival patients were consecutively enrolled and biochemical parameters were collected at three time points: (1) the day of hospitalization due to the respiratory symptoms onset (T0); (2) day 3 (T3) and day 7 (T7) after the admission. At the same time points, arterial blood gas was performed to evaluate the respiratory function based on partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2 ratio).
Out of the 153 patients, three were drop-outs. Specifically, one of them refuses to keep on participating, and two were suspected to have a neoplastic disease. Of the remaining 150 patients, 94, who presented with mild COVID-related symptoms, were assigned to population “A” and 56 with severe COVID-19-related symptoms to population “B”, according to the global clinical status. In particular, patients were divided in two reference populations using the term “A” or “B”, if the evaluation of the WHO score at T7, was between 1 and 4 or between 5 and 8, respectively. The fundamental criterion to be part of the population “A” or “B” was the need or not of oxygen support by the infected patients.
In particular, the World Health Organization (WHO) was engaged to classify the severity of this infection for COVID-19 trial endpoints (http://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/). The WHO ordered the severity of the infectious disease in “low” (WHO score 1–2) that correspond to the presence of mild symptoms, “moderate” for patients who no required supplemental oxygen (WHO scores 3–4), and “severe” for all patients who needed oxygen support (WHO scores 5–8 high-flow oxygen requirement continuous positive airway pressure (CPAP), non-invasive ventilation (NIV), multi-organ support and death).
Exclusion criteria
Patients were excluded if they were known to be pregnant, had a history of vasculitis or connective tissue disease and were subjected to long-term corticosteroids or chronically immunosuppressed, received antivirals, anti-interleukin (IL)-1, anti-IL-6 or anti-TNF therapy, or underwent dialysis for chronic kidney disease or had suspect/active neoplasia.
Predictive index test
As predictive index test was used the PaO2/FiO2 ratio, the most reliable diagnostic criteria for acute lung injury and ARDS [16].
Principal outcome
As principal outcome, we wondered to investigate if PaO2/FiO2 ratio was reliable for classifying SARS-CoV-2-infected patients who will show a severe disease.
Secondary outcome
We were interested in comparing the predictive value of PaO2/FiO2 ratio to inflammatory scores (hs-CRP, NLR, PLR and LDH), already known to be useful in predicting the outcome of SARS-CoV-2-infected patients.
Validation cohort
To validate PAO2/FiO2 ratio as a reliable COVID-19 risk biomarker for the identification of patients who will develop critical illness, according to the WHO criteria, we retrospectively analyzed the clinical and biochemical data of 170 patients (broken into Population “A” and “B”) collected by two hospitals of central Italy. The variables required for validating PaO2/FiO2 ratio were collected and cross-checked by two experienced physicians (Table S2).
The number of patients, both in the discovery and in the validation cohort, who were given oxygen support is shown in Table S3.
Statistical evaluation
Variables were not normally distributed and were expressed as median plus 1st and 3rd interquartile, after controlling by the Shapiro–Wilk test. Difference in medians was evaluated by the Mann–Whitney U test. Frequencies were analyzed by the chi-square.
As measure of association, we chose to study predictions that were carried out by two types of regression techniques.
Dealing with the binary dependent variable or dichotomous outcome, i.e., Population “A”/“B” at time point T7, and one independent variable the simple logistic regression was used, calculating the odds ratio, the Std. Err. the z, the P and 95% confidence intervals (CI).
In case of more than one independent variable, the multiple logistic regression was performed, always calculating the same afore mentioned parameters, by using the enter method alone. This choice was based on having strong hypotheses about which variables belong in the model. There were implanted three models, i.e., model A comprehending all the inflammatory parameters, model B adjusted for PaO2/FiO2 ratio and model C, further adjusted for age.
ROC analysis was used as a diagnostic decision-making. Indicatively, to measure the performance of the binary classification test (index test) was studied the best cut-off, coupled with the sensitivity, specificity, positive likelihood ratio (LR +) = sens/(1-spec) and the negative likelihood ratio (LR-) = (1-sens)/spec), pointing out that the more the LR + is > 1, the more likely the outcome. On the contrary, the more a likelihood ratio for a negative test is < 1, the less likely the outcome.
Furthermore, the correct classification percentage of Population “A”/“B” at the time point T7 and the area under the receiver operating characteristic (AUROC/AUC) were performed to evaluate the most appropriate models (the highest specificity and sensitivity), under the nonparametric assumption. The cut-off (cut-points) with the highest specificity and sensitivity was calculated by the means of Youden’s Index according to [17].
Results
Dynamic profile of SARS-CoV-2-infected patients
Of the whole cohort of patients 38% were female, the mean age was 61 (ranging from 51.75 to 70.25). Among all the parameters analyzed the following displayed statistically significant changes during the first week of hospitalization: PaO2/FiO2 ratio, HR, procalcitonin, D-dimer, INR, ALT, AST, gamma-GT, FiO2, hs-CRP, platelet and lymphocyte count (Table 1).
ALT (T0 vs T3, P < 0.01 and T3 vs T7, P < 0.01), AST (T0 vs T3, P < 0.01) and gamma-GT (T0 vs T3, P < 0.05 and T3 vs T7, P < 0.05) were significantly increased in our cohort of patients during the progression of the infection.
Among the respiratory function parameters, FiO2 and PaO2/FiO2 ratio displayed significant negative trend from T0 to T3 (P < 0.001 and P < 0.001, respectively), that appeared to be significantly improved at day 7 (P < 0.001 and P < 0.05, respectively).
Platelet and lymphocyte count were significantly increased from T3 to T7 (P < 0.001 and P < 0.05, respectively).
Characteristics of the whole population and of both cohorts “A” and “B”
The demographics and clinical characteristics are shown in Table 2.
The rate of severe (population “B”) and moderate cases (population “A”) was 37.4% and 62.6%, respectively.
The population “B” was characterized by the presence of older patients respect to the population “A” (median 57.50 vs 68.00, P < 0.001), without any difference between cohorts concerning gender (chi square, P = 0.65). Fever (86%) and cough (71.3%) were the first and most common symptoms at admission, and, in this regard, no significant differences between the two populations were observed. RR was higher in the population “B” [21 (17;28) vs 17 (15;19), P < 0.05].
The length of stay of the whole cohort of patients was 12 days (7.25–19.00), population A and population B length stay were 8.5 (6.00–13.00) and 18 (11.25–26.00) days, respectively (Table 2).
Most severe cases (population “B”) exhibited higher rate of co-morbidities (58.93 vs 38.30%) without reaching significance. The rate of patients worsening to ARDS was 38.6% and mortality was 9.3%.
Procalcitonin, ferritin, fibrinogen, D‐dimer, international normalized ratio (INR) and alkaline phosphatase (ALP) were significantly higher in the population “B” respect to “A” (P < 0.001, P < 0.01, P < 0.05, P < 0.001, P < 0.01, P < 0.001, respectively), whereas the liver functional parameters were not significantly different between the two populations.
Lymphocytes [median 0.970 (0.660–1.335)], neutrophil [4.32 (2.83–6.56)] and platelet [196.0 (154.5–254.0)] were collected in whole patients and significant differences in neutrophil and platelet count were observed in the population “B” respect to “A” [median 227 (146.5–294.8) and 261 (194–309), P < 0.001, respectively].
PaO2/FiO2 ratio versus LDH, hs-CRP, NLR and PLR in the population “A” and “B”
Logistic regression analysis revealed a significant negative prediction of the clinical outcome at day 7 by PaO2/FiO2 ratio (P < 0.001).
Similarly, LDH, hs-CRP, and PLR were positively correlated with a poor outcome (P < 0.001, P < 0.001, P = 0.016, respectively) (Table S1).
To evaluate the predictive power of the PaO2/FiO2 ratio, we performed three models of multivariable regression analysis.
Among the three different models, the model A was performed to correlate the inflammatory parameters (hs-CRP, NLR, PLR and LDH) at the time of hospitalization with the clinical outcome at T7. Our analyses showed that the most representative variables were LDH and hs-CRP (P = 0.002 and P = 0.039, respectively) (Table 3). Surprisingly, when we adjusted the model for PaO2/FiO2 ratio (model B, Table 3), we observed a completely different scenario, letting us think that the predictive ability of the respiratory function parameter likely obscures the prognostic value of the inflammation parameters (Table 3). To confirm the result of model B we performed a multivariable regression analysis adjusted for age (model C, Table 3).
Remarkably, when we analyzed age, at univariate analysis, as a single predictor, it resulted very significant (P < 0.001, Table S1).
Developing a clinical prediction model
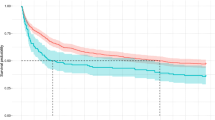
Dealing with the diagnostic accuracy of an index test, we generate the AUC curves of all the studied parameters. AUC curve of PaO2/FiO2 ratio for differentiating the population “A” and “B”, which was 0.838, (95% CI 0.771–0.908). The PaO2/FiO2 appeared as the most reliable prognostic biomarker compared to hs-CRP, NLR, PLR and LDH (AUC curves of 0.725, 0.741, 0.632, 0.729, respectively), see Fig. 1.
The optimum PaO2/FiO2 ratio cut-off value to separate population “A” from “B” was < 274 mmHg, with sensitivity and specificity of 71.79% and 85.25% (LR + 4.8661, LR−0.3309), respectively.
Model validation
To establish its reproducibility, we tested the reliability of the PaO2/FiO2 ratio in the validation cohort, including retrospectively 170 patients, 100 were female, with a median age of 68.00 years (Table S2). The AUC of the PaO2/FiO2 ratio resulted to be 0.826 (95% CI 0.760–0.891), Fig. 2. The cut-off neared that was found in the discovery cohort with sensitivity of 82.50% and specificity 71.74%, LR + 2.9192, LR−0.2439.
Discussion
Summarizing the key results, our research highlights that PaO2/FIO2 ratio could be considered a reliable prognostic biomarker for patients’ management of COVID-19 pneumonia, as demonstrated by the AUC curve that indicate as cut-off the value < 274 mmHg. The performance of this biomarker was more than satisfactory with a good accuracy based on AUCs, in both the discovery and validation cohorts, of 0.838 and 0.826, respectively.
It is known that COVID-19 prognosis is worse in older patients, but in an interesting way, age did not modify the prediction by PaO2/FiO2 ratio. Presenting the importance of the study, we think that one of the strengths of our work is that we tracked levels of biomarkers with the aim to establish trends for each patient towards the successive phases of the disease.
The likely contribution of this work to literature consists in evidencing that in our cohort of patients, among the variables analyzed (PaO2/FiO2 ratio, hs-CRP, NLR, PLR and LDH), PaO2/FiO2 ratio was the best independent prognostic biomarker for forecasting pneumonia progression toward ARDS in COVID-19 patients.
Describing similar data of literature, our findings were consistent with those of previous studies on LDH, hs-CRP, NLR, PLR [1, 8,9,10,11,12] that associated the afore mentioned prognostic markers with poor disease outcome. However, the reliability of PaO2/FiO2 ratio in comparison with other inflammatory tests to forecast the worst outcomes of COVID-19-positive patients has not yet been highlighted.
Various indices have been used to describe pneumonia progression towards ARDS, such as the arterial to alveolar O2 difference, the intrapulmonary shunt fraction, the oxygen index and the PaO2/FIO2 ratio [18]. Of these different indices, the PaO2/FIO2 ratio, because of its accuracy but also its simplicity, could be taken into serious account also by hospitalists in their every-day practice when dealing with OVID-19.
Concerning this specific pulmonary test, Grasselli et al. in 1591 patients, admitted to an intensive care unit (ICU), found a reduced median of 160 mmHg (IQR 114–220) [19]. According to our results, Santus et al. discovered that a moderate-to-severe impairment in PaO2/FiO2 ratio was independently associated with a threefold increase in risk of intra-hospital mortality, concluding that the severity of respiratory failure is useful to identify patients at higher risk of mortality [20].
Our study covered a time period during which advances and changes in treatment approaches may have differently reduced the levels of inflammatory biomarkers among patients hospitalized with COVID-19, but this aspect does not affect the importance of our index parameter, on the contrary it reinforces its reliability.
Conclusion
Could our results fill existing gaps in the field that had not been previously exposed? We aimed to give to clinicians a way to discriminate SARS-CoV-2-positive patients with different fate. We intentionally categorized our cohort in “low” (population “A”) or “high” (population “B”)-risk patients in such a way that clinicians could make decisions quickly based on local or regional conditions and opportune therapeutic approaches. In fact, we consider of great importance the access to good clinical and supportive care for providing a more or less aggressive care whereby the patient’s outcomes might be optimized.
Limitations
Possible limitations exist in this paper. The study was conducted with a not so large sample size and on data deriving only from the central Italy, which could limit the value of PaO2/FiO2 ratio as a prognostic biomarker representative of a general population.
Moreover, although the PaO2/FiO2 ratio is simple and easily available, it has a suboptimal power in categorizing patients with ARDS [21].
Future directions
As work in progress, we will follow up these cohorts of patients. Nowadays, everlasting effects of COVID-19 are still largely unknown. Long-term pulmonary and systemic complications have been reported in some patients after recovery, with residual fatigue or muscle weakness, chest CT abnormalities, cardiovascular and neurological complications [22, 23].
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute distress respiratory syndrome
- PaO2 :
-
Partial pressure of arterial oxygen
- FiO2 :
-
Fraction of inspired oxygen
- hs-CRP:
-
High-sensitivity C-reactive protein
- NEU:
-
Neutrophil
- LYM:
-
Lymphocyte
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PLT:
-
Platelet
- PLR:
-
Platelet-to-lymphocyte ratio
- LDH:
-
Lactate dehydrogenase
- CPAP:
-
Continuous positive airway pressure
- NIV:
-
Non-invasive ventilation
- LR:
-
Likelihood ratio
- INR:
-
International normalized ratio
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- GGT:
-
Gamma-glutamyl transferase
- HR:
-
Heart rate
- RR:
-
Respiratory rate
References
Guan W, Ni Z, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1016/j.jemermed.2020.04.004
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc 323(13):1239–1242. https://doi.org/10.1001/jama.2020.2648
Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, Klauber J, Janssens U, Marx G, Weber-Carstens S, Kluge S, Pfeifer M, Grabenhenrich L, Welte T, Busse R (2020) Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 8(9):853–862. https://doi.org/10.1016/S2213-2600(20)30316-7
Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, Huet K, Plzak J, Horoi M, Hans S, Rosaria Barillari M, Cammaroto G, Fakhry N, Martiny D, Ayad T, Jouffe L, Hopkins C, Saussez S, COVID-19 Task Force of YO-IFOS (2020) Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med 288(3):335–344. https://doi.org/10.1111/joim.13089
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS, ARDS Definition Task Force (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307(23):2526–2533. https://doi.org/10.1001/jama.2012.5669
Matricardi P, Dal Negro R, Nisini R (2020) The first, holistic immunological model of COVID-19: implications for prevention, diagnosis, and public health measures. Paediatr Allergy Immunol 31(5):454–470. https://doi.org/10.1111/pai.13271
Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM (2020) Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 20(6):669–677. https://doi.org/10.1016/S1473-3099(20)30243-7
Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, Sanderson C, McKee M, Troeger C, Ong KL, Checchi F, Perel P, Joseph S, Gibbs HP, Banerjee A, Eggo RM, Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group (2020) Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health 8(8):e1003–e1017. https://doi.org/10.1016/S2214-109X(20)30264-3
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Betakova T, Kostrabova A, Lachova V, Turianova L (2017) Cytokines induced during influenza virus infection. Curr Pharm Des 23(18):2616–2622. https://doi.org/10.2174/1381612823666170316123736
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
Yang AP, Liu JP, Tao WQ, Li HM (2020) The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. https://doi.org/10.1016/j.intimp.2020.106504
Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, Deng G (2020) Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis 96:467–474. https://doi.org/10.1016/j.ijid.2020.05.055
Li C, Ye J, Chen Q, Hu W, Wang L, Fan Y, Lu Z, Chen J, Chen Z, Chen S, Tong J, Xiao W, Mei J, Lu H (2020) Elevated lactate dehydrogenase (LDH) level as an independent risk factor for the severity and mortality of COVID-19. Aging (Albany NY) 12(15):15670–15681. https://doi.org/10.18632/aging.103770
Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, Lippi G (2020) Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med 38(9):1722–1726. https://doi.org/10.1016/j.ajem.2020.05.073
Broccard AF (2013) Making sense of the pressure of arterial oxygen to fractional inspired oxygen concentration ratio in patients with acute respiratory distress syndrome. OA Critical Care 1(1):9
Fluss R, Faraggi D, Reiser B (2005) Estimation of the Youden Index and its associated cut-off point. Biom J 47(4):458–472. https://doi.org/10.1002/bimj.200410135
Lenti MV, de BorrelliAndreis F, Pellegrino I, Klersy C, Merli S, Miceli E, Aronico N, Mengoli C, Di Stefano M, Cococcia S, Santacroce G, Soriano S, Melazzini F, Delliponti M, Baldanti F, Triarico A, Corazza GR, Pinzani M, Di Sabatino A (2020) Impact of COVID-19 on liver function: results from an internal medicine unit in Northern Italy. Intern Emerg Med 15(8):1399–1407. https://doi.org/10.1007/s11739-020-02425-w
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, COVID-19 Lombardy ICU Network (2020) Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy regionitaly. JAMA 323(16):1574–1581. https://doi.org/10.1001/jama.2020.5394
Santus P, Radovanovic D, Saderi L, Marino P, Cogliati C, De Filippis G, Rizzi M, Franceschi E, Pini S, Giuliani F, Del Medico M, Nucera G, Valenti V, Tursi F, Sotgiu G (2020) Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: a prospective observational multicentre study. BMJ Open. https://doi.org/10.1136/bmjopen-2020-043651
Lanspa MJ, Morris AH (2015) The PaO2/FIO2 ratio categorization of patients with acute respiratory distress syndrome is suboptimal. Crit Care Med 43(2):488–489. https://doi.org/10.1097/CCM.0000000000000816
Salehi S, Reddy S, Gholamrezanezhad A (2020) Long-term Pulmonary consequences of coronavirus disease 2019 (COVID-19): what we know and what to expect. J Thorac Imaging 35(4):W87–W89. https://doi.org/10.1097/RTI.0000000000000534
Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T (2020) Neurological associations of COVID-19. Lancet Neurol 19(9):767–783. https://doi.org/10.1016/S1474-4422(20)30221-0
Funding
Open access funding provided by Università degli Studi dell’Aquila within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
CB conceptualized and designed the study, carried out statistical analyses and drafted the manuscript. GS and SJS collected clinical data, carried out the data and statistical analyses, and reviewed and revised the manuscript. GT carried out the data and statistical analyses, did interpretation of data and critical revision for important intellectual content. GP, BC, FR, NC, MAZ, NI, SB, AA, PC, MRC, CMM and AG enrolled patients, coordinated and supervised data collection. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the institutional ethic committee (License prot. n. 71726).
Statement of human and animal rights
The study was approved by the local ethical committee.
Consent to participate
Informed consent was obtained from participating patients at the San Salvatore Hospital, L’Aquila, Italy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sinatti, G., Santini, S.J., Tarantino, G. et al. PaO2/FiO2 ratio forecasts COVID-19 patients’ outcome regardless of age: a cross-sectional, monocentric study. Intern Emerg Med 17, 665–673 (2022). https://doi.org/10.1007/s11739-021-02840-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-021-02840-7