Abstract
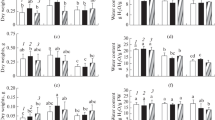
This study reports a comparison of differential physiological and biochemical changes in two Indian Mustard (Brassica juncea L.) cultivars viz. CS-52 (salinity tolerant) and Ashirwad (salinity susceptible) after 15 days of gradual increase in NaCl concentration in the nutrient solution. The increase in the NaCl concentration in the nutrient solution was as follows: 25 mM for 2 days, 50 mM for 2 days, 75 mM for 2 days, 100 mM for 2 days, 125 mM for 2 days, and 150 mM for 5 days. After 15 days of salinity stress, we observed a sharp decline in dry matter content and leaf area in Ashirwad. These effects were, however, less pronounced in CS-52. Under high salinity conditions, CS-52 maintained a better physiological status as determined by higher relative water content, higher water use efficiency, and lower leaf temperature and electrolytic leakage ratio, compared to Ashirwad. CS-52 was also observed to be more efficient regarding gas-exchange parameters (stomatal conductance and transpiration) and photosynthetic capacity. Moreover, salt-induced changes in accumulation and distribution patterns, and the ratios of major macro- and microelements were recorded to be more favorable in CS-52 compared to Ashirwad. The study also revealed that salinity-induced relative changes in the concentrations and compositions of biomolecules such as lipids, proteins, and carbohydrates, and structural rearrangements in the side chains of proteins were less prominent in CS-52 indicating better preparedness and thus more adaptability of CS-52 towards salinity.














Similar content being viewed by others
References
Al-Karaki GN (2006) Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci Hort 109:1–7
Aziz EE, Gad N, Khaled SM (2011) Effect of cobalt on growth and chemical composition of peppermint plant grown in newly reclaimed soil. Aust J Basic Appl Sci 5:628–633
Botella MA, Martinez V, Pardines J, Cerda A (1997) Salinity induced potassium deficiency in maize plants. J Plant Physiol 150:200–205
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Chakraborty K, Sairam RK, Bhattacharya RC (2012) Differential expression of salt overly sensitive pathway genes determines salinity stress tolerance in Brassica genotypes. Plant Physiol Biochem 51:90–101
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Cooil BJ, De La Fuente RK, De La Pena RS (1965) Absorption and transport of sodium and potassium in squash. Plant Physiol 40:625–633
Cramer GR (2004) Sodium-calcium interactions under salinity stress. In: Läuchli A, Lütge U (eds) Salinity: environment-plants-molecules. Kluwer Academic, New York, pp 205–228
Davies KJ (1987) Protein damage and degradation by oxygen radicals. I. General aspects. J Biol Chem 262:9895–9901
Demiral T, Türkan İ (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257. https://doi.org/10.1007/s00709-017-1159-z
El-Gamal IM (2000) Interaction between cobalt and salinity on the plant growth. ICEHM2000, Cairo Univ., Egypt
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Fridovich I (1986) Superoxide dismutases. In: Meister A (ed) Advances in enzymology and related areas of molecular biology. Wiley, Hoboken, pp 61–97
Giri B, Kapoor R, Mukerji KG (2003) Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol Fertil Soils 38:170–175
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Grattan SR, Grieve CM (1999) Mineral nutrient acquisition and response by plants grown in saline environments. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 203–229
Hayat S, Mir BA, Wani AS, Hasan SA, Irfan M, Ahmad A (2011) Screening of salt-tolerant genotypes of Brassica juncea based on photosynthetic attributes. J Plant Interact 6:53–60
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition, Technical Communication No. 22, Commonwealth Agricultural Bureaux. Eastern Press, London
Hirpara KD, Ramoliya PJ, Patel AD, Pandey AN (2005) Effect of salinization of soil on growth and macro- and micro-nutrient accumulation in seedlings of Butea monosperma (Fabaceae). Ann Biol 27:3–14
Islam F, Yasmeen T, Ali S, Ali B, Farooq MA, Gill RA (2015) Priming-induced antioxidative responses in two wheat cultivars under saline stress. Acta Physiol Plant 37:153
Islam F, Ali B, Wang J, Farooq MA, Gill RA, Ali S, Wang D, Zhou W (2016a) Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol Biochem 107:82–95
Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S, Zhou W (2016b) Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul 80:23–36
Islam F, Farooq MA, Gill RA, Wang J, Yang C, Ali B, Wang G, Zhou W (2017a) 2,4-D attenuates salinity-induced toxicity by mediating anatomical changes, antioxidant capacity and cation transporters in the roots of rice cultivars. Sci Rep 7:10443
Islam F, Xie Y, Farooq MA, Wang J, Yang C, Gill RA, Zhu J, Zhou W (2017b) Salinity reduces 2,4-D efficacy in Echinochloa crusgalli by affecting redox balance, nutrient acquisition, and hormonal regulation. Protoplasma 255:785–802
Kizil R, Irudayaraj J, Seetharaman K (2002) Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J Agric Food Chem 50:3912–3918
Kopittke PM, Menzies NW (2005) Effect of pH on Na induced calcium deficiency. Plant Soil 269:119–129
Mahajan S, Tuteja N (2005) Cold, salinity, and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Mandhania S, Madan S, Sawhney V (2006) Antioxidant defense mechanism under salt stress in wheat seedlings. Biol Plant 50:227–231
Naumann D, Helm D, Labischinski H, Giesbrecht P (1991) The characterization of microorganisms by Fourier-transform infrared spectroscopy (FT–IR). In: Nelson WH (ed) Modern techniques for rapid microbiological analysis. VCH, New York, pp 43–96
Noreen Z, Ashraf M, Akram NA (2010) Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J Agron Crop Sci 196:273–285
Olias R, Eljakaoui Z, Li J, De Morales PA, Marin-Manzano MC, Pardo JM, Belver A (2009) The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ 32:904–916
Robinson SP, Downton WJS, Millihouse JA (1983) Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach. Plant Physiol 73:238–242
Shabala S (2009) Salinity and programmed cell death: unravelling mechanisms for ion specific signaling. J Exp Bot 60:709–712
Sharma P, Sardana V, Banga SS (2013) Salt tolerance of Indian mustard (Brassica juncea) at germination and early seedling growth. Environ Exp Biol 11:39–46
Shekhawat K, Rathore SS, Premi OP, Kandpal BK, Chauhan JS (2012) Advances in agronomic management of Indian mustard (Brassica juncea (L.) Czernj. Cosson.): an overview. Int J Agron 22:22. https://doi.org/10.1155/2012/408284
Shoresh M, Spivak M, Bernstein N (2011) Involvement of calcium-mediated effects on ROS metabolism in the regulation of growth improvement under salinity. Free Radic Biol Med 51:1221–1234
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Stehfest K, Toepel J, Wilhelm C (2005) The application of micro-FTIR spectroscopy to analyze nutrient stress-related changes in biomass composition of phytoplankton algae. Plant Physiol Biochem 43:717–726
Surewicz WK, Mantsch HH (1988) New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta 952:115–130
Szollosi R (2011) Indian Mustard (Brassica juncea L.) seeds in health. In: Preedy V, Watson R, Patel V (eds) Nuts and seeds in health and disease prevention. Academic, New York, pp 671–676
Tozlu I, Moore GA, Guy CL (2002) Effects of increasing NaCl concentration on stem elongation, dry mass production, and macro- and micro-nutrient accumulation in Poncirustri foliata. Aust J Plant Physiol 27:35–42
Ueda A, Kathiresan A, Inada M, Narita Y, Nakamura T, Shi W, Takabe T, Bennett J (2004) Osmotic stress in barley regulates expression of a different set of genes than salts tress does. J Exp Bot 406:2213–2218
Welch RM, Graham RD (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55:353–364
Wu J, Vincent B, Yang J, Bouarfa S, Vidal A (2008) Remote sensing monitoring of changes in soil salinity: a case study in Inner Mongolia, China. Sensors (Basel, Switzerland) 8:7035–7049
Yang J, Yen HE (2002) Early salt stress effects on the changes in chemical composition in leaves of ice plant and Arabidopsis. A Fourier transform infrared spectroscopy study. Plant Physiol 130:1032–1042
Yermiyahu U, Ben-Gal A, Keren R, Reid RJ (2008) Combined effect of salinity and excess boron on plant growth and yield. Plant Soil 304:73–87
Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV (2010) The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22:4128–4141
Acknowledgements
We sincerely acknowledge Director, ICAR-Directorate of Rapeseed-Mustard Research, Bharatpur, 321 303, Rajasthan (India), for providing financial support and the facilities to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by W. Zhou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, B.K., Singh, S.P., Shekhawat, K. et al. Comparative analysis for understanding salinity tolerance mechanism in Indian Mustard (Brassica juncea L.). Acta Physiol Plant 41, 104 (2019). https://doi.org/10.1007/s11738-019-2894-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2894-x