Abstract
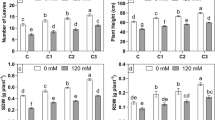
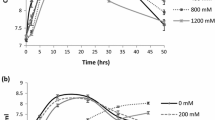
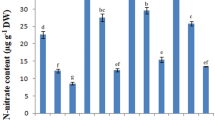
Salt stress has multiple damaging effects on plants including physiological damage, reduced growth, and productivity. Plant growth-promoting rhizobacteria (PGPR) are one of the valuable options to mitigate the negative effects of this stress. In the present study, native bacteria from chickpea’s rhizosphere were isolated, and checked for their salt tolerance and plant growth-promoting attributes (phosphate (P) solubilization, siderophores, indole-3-acetic acid (IAA) production, and 1-aminocyclopropane-1-carboxylate (ACC) deaminase production). One isolate, subsequently identified as Pantoea dispersa, showed appreciable production of IAA (218.3 µg/ml) and siderophores (60.33% SU), P-solubilization (3.64 µg/ml) and ACC deaminase activity (207.45 nmol/mg/h) in the presence of 150 mM NaCl, under laboratory conditions. Salt stress in uninoculated chickpea (GPF2 cultivar) plants induced high accumulation of Na+ ions (3.86 mg g−1 dw) in the leaves, along with significant reduction in K+ uptake, membrane integrity, chlorophyll concentration, and leaf water content, thus resulting in impaired growth of the plant and yield (pods and seeds) in a salt concentration-dependent manner. The damage due to salt stress was restored significantly in plants inoculated with P. dispersa. A significant improvement in biomass (32–34%), pods number (31–34.5%), seeds number (32–35.7%), pods weight (30–32.6%), and seeds weight (27–35%) per plant occurred in salt stress-affected plants, which was associated with significant reduction in Na+ uptake, reduced membrane damage, significantly improved leaf water content, chlorophyll content, and K+ uptake. This study suggests for the first time that native P. dispersa strain PSB3 can be used to alleviate the negative effects of salt stress on chickpea plants and holds the potential to be used as a biofertilizer.



Similar content being viewed by others
References
Aamir M, Aslam A, Khan MY, Jamshadi MU, Ahmed M, Asghar HN, Zahir ZA (2013) Co-inoculation with Rhizobium and plant growth promoting rhizobacteria (PGPR) for inducing salinity tolerance in mungbean under field condition of semi-arid climate. Asian J Agri biol 1:17–22
Amellal N, Burtin G, Bartoli F, Heulin T (1998) Colonization of wheat roots by EPS-producing Pantoea agglomerans and its effect on rhizosphere soil aggregation. Appl Environ Microbiol 64:3740–3747
Amor AF, Cuadra-Crespo A (2012) Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Func Plant Biol 39:82–90
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashraf M, Berge SH, Mahmood OT (2004) Inoculating wheat seedling with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soil 40:157–162
Barbhaiya HB, Rao KK (1985) Production of pyoverdine the fluorescent pigments of Pseudomonas aeruginosa PAO1. FEMS Microbiol Lett 27:233–235
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Betancor L, Schelotto F, Martinez A, Pereira M, Algorta G, Rodriguez MA, Vignoli R, Chabalgoity JA (2004) Random Amplified Polymorphic DNA and phenotyping analysis of Salmonella enterica serovar Enteritidis isolates collected from humans and poultry in Uruguay from 1995 to 2002. J Clin Microbiol 42:1155–1162
Betancor L, Pereira M, Martinez A, Giossa G, Fookes M, Flores K, Barrios P, Repiso V, Vignoli R, Cordeiro N, Algorta G, Thomson N, Maskell D, Schelotto F, Chabalgoity JA (2010) Prevalence of Salmonella enterica in poultry and eggs in Uruguay during an epidemic due to Salmonella enterica serovar Enteritidis. J Clin Microbiol 48:2413–2423
Bradford MM (1976) A rapid and sensitive method for quantification of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Breed RS, Murray EGD, Smith NR (1957) Bergey’s manual of determinative bacteriology, 7th edn. Williams and Wilkins, Baltimore, pp 332–334
Chauhan PS, Nautiyal CS (2010) The purB gene controls rhizosphere colonization by Pantoea agglomerans. Lett Appl Microbiol 50:205–210
Egamberdieva D, Berg G, Lindstrom K, Rasanen LA (2013) Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil. doi:10.1007/s11104-013-1586-3
Frankenberger WT Jr, Poth M (1988) L-tryptophan transaminase of a bacterium isolated from rhizosphere of Festuca octoflora. Soil Biol Biochem 20:299–304
Fuentes-Ramirez LE, Caballero-Mellado J (2006) Bacterial biofertilizers. In: Siddiqui ZA (ed) PGPR: Biocontrol and biofertilization. Springer, Dordechet, pp 143–172
Gorden SA, Paleg LG (1957) Observations on the quantitative determination of indole acetic acid. Plant Physiol 10:39–47
Holiday ER, Preedy JRK (1953) Precision of a direct reading flame photometer for the determination of sodium and potassium in biological fluids. Biochem J 55:214–220
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831
Hussain K, Ashraf M, Ashraf MY (2008) Relationship between growth and ion relation in pearl millet (Pennisetum glaucum L.) at different growth stages under salt stress. Afr J Plant Sci 2:23–27
Jackson ML (1973) Methods of chemical analysis. Prentice hall of India (Pvt) Ltd, New Delhi
Kang S, Khan AL, Waqas M, You Y, Kim JH, Kim JG, Humayun M, Lee I (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9:673–682
Karlidag H, Yildrim E, Turan M, Pehluvan M, Donmez F (2013) Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria ananassa). HortScience 48:563–567
Kovac N (1956) Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature London 178:703
Laslo E, Gyorgy E, Mara G, Tamas E, Abraham B, Lanyi S (2012) Screening of plant growth promoting rhizobacteria as potential microbial inoculants. Crop Protection 40:43–48
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
MacFaddin JF (2000) Biochemical tests for identification of medical bacteria, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Mathivanan S, Chidambaram ALA, Sundramoorthy P, Baskaran L, Kalaikandhan R (2014) Effect of combined inoculations of plant growth promoting rhizobacteria (PGPR) on the growth and yield of groundnut (Arachis hypogaea L.). Int J Curr Microbiol Appl Sci 3:1010–1020
Minaxi LN, Yadav RC, Saxena J (2012) Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl Soil Ecol 59:124–135
Mishra A, Chauhan PS, Chaudhary V, Tripathi M, Nautiyal CS (2011) Rhizosphere competent Pantoea agglomerans enhances maize (Zea mays) and chickpea (Cicer arietinum L.) growth, without altering the rhizosphere functional diversity. Antonie Van Leeuwenhoek 100:405–413
Moller V (1955) Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Path Microbiol Scand 36:158–172
Nadeem SM, Zahir ZA, Naveed M, Nawaz S (2013) Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann Microbiol 63:225–232
Oliveira ABD, Alencar NLM, Gomes-Filho E (2013) Comparison between the water and salt stress effects on plant growth and development. In: Sener A (ed) Responses of organisms to water stress. InTech, Rijeka, pp 67–94
Ondrasek G, Rengel Z, Veres S (2011) Soil salinization and salt stress in crop production. In: Shanker A (ed) Abiotic stress in plants—mechanisms and adaptations. InTech, Rijeka, pp 171–190
Panwar M, Tewari R, Nayyar H (2014) Microbial consortium of plant growth promoting rhizobacteria to enhance performance of plants growing in stressed-soils: an overview. In: Khan MS, Zaidi A, Musarrat J (eds) Phosphate solubilizing microorganisms. Springer International Publishing, Berlin, pp 257–285
Paul S, Bandeppa Aggarwal C, Thakur JK, Rathi MS, Khan MA (2014) Effect of salt on growth and plant growth promoting activities of Azotobacter chroococcum isolated from saline soil. Environ Ecol 32:1255–1259
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase containing plant growth promoting rhizobacteria. Physiol Plant 118:10–15
Reetha S, Bhuvaneswari G, Thamizhiniyan P, Mycin TR (2014) Isolation of indole acetic acid (IAA) producing rhizobacteria of Pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allium cepa L.). Int J Curr Microbiol Appl Sci 3:568–574
Sarkar A, Patel JS, Yadav S, Sharma BK, Srivastva JS, Singh HB (2014) Studies on rhizosphere-bacteria mediated biotic and abiotic stress tolerance in chickpea (Cicer arietinum L.). Vegetos 27:158–169
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sergeeva E, Hirkala DLM, Nelson LM (2007) Production of indole-3-acetic acid, aromatic amino acid amino transferase activities and plant growth promotion by Pantoea agglomerans rhizosphere isolates. Plant Soil 297:1–13
Shahzad SM, Khalid A, Arshad M, Tahir J, Mahmood T (2010) Improving nodulation, growth and yield of Cicer arietinum L. through bacterial ACC-deaminase induced changes in root architecture. Euro J Soil Biol 46:342–347
Shrivasatava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Shukla PS, Agarwal PK, Jha B (2012) Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant growth promoting rhizobacteria. J Plant Growth Regul 31:195–206
Singh O, Gupta M, Mittal V, Kiran S, Nayyar H, Gulati A, Tewari R (2014) Novel phosphate solubilizing bacteria Pantoea cypripedii PS1 along with Enterobacter aerogenes PS16 and Rhizobium ciceri enhance the growth of chickpea. Plant Growth Regul 73:79–89
Taiz L, Zeiger E (2002) Plant physiology, 3rd edn. Sinauer Associates, Sunderland
Taylor WI, Achanzar D (1972) Catalase test as an aid in identification of Enterobacteriaceae. Appl Microbiol 24:58–61
Turki Y, Mehri I, Fhoula I, Hassen A, Ouzari H (2014) Comparison of five molecular subtyping methods for differentiation of Salmonella Kentucky isolates in Tunisia. World J Microbiol Biotechnol 30:87–98
Upadhyay SK, Singh DP (2014) Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol 17:288–293
Verma JP, Yadav J, Tiwari KN, Kumar A (2013) Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol Eng 51:282–286
Verma JP, Yadav J, Tiwari KN, Jaiswal DK (2014) Evaluation of plant growth promoting activities of microbial strains and their effect on growth and yield of chickpea (Cicer arietinum L.) in India. Soil Biol Biochem 70:33–37
Weisberg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Winn WC Jr, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P (2006) Koneman’s color atlas and textbook of diagnostic microbiology, 6th edn. Lippincott Williams and Wilkins, Philadelphia, p 1565
Yadav S, Irfan M, Ahmed A, Hayat S (2011) Causes of salinity and plant manifestations of salt stress: a review. J Environ Biol 32:667–685
Younesi O, Chaichi MR, Postini K (2013) Salt tolerance in alfalfa following inoculation with Pseudomonas. Middle-East J Sci Res 16:101–107
Acknowledgements
We sincerely thank Council of Scientific and Industrial Research, India, for providing the financial assistance to the first author and Dr. Arvind Gulati from Institute of Himalayan Bioresource Technology (CSIR), Palampur, Himachal Pradesh, India, for identification of strains using 16S rRNA gene sequencing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zwiazek.
Rights and permissions
About this article
Cite this article
Panwar, M., Tewari, R., Gulati, A. et al. Indigenous salt-tolerant rhizobacterium Pantoea dispersa (PSB3) reduces sodium uptake and mitigates the effects of salt stress on growth and yield of chickpea. Acta Physiol Plant 38, 278 (2016). https://doi.org/10.1007/s11738-016-2284-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2284-6