Abstract
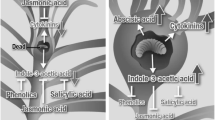
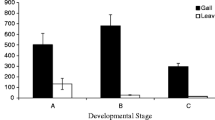
The fluid-feeding aphid Schlechtendalia chinensis (Bell) induces horned galls on its primary host, the Chinese Sumac (Rhus chinensis Mill). Horned galls are harvested for their high content of tannins, and used in a range of medical and chemical applications. Gall development is a complex and highly controlled physiological process, where the growing insect population manipulates the plant developmental programs that allow the transformation of plant tissue into a gall. In this study, we examine whether Schlechtendalia alters the balance of plant hormones in the host tree as a means to achieve gall formation. For this, we measured concentrations for a series of endogenous hormones, including indole-3-acetic acid (IAA), cytokinin (CTK), gibberellic acid (GA), abscisic acid (ABA), jasmonic acid (JA), salicylic acid (SA), and ethylene (ETH). Specifically, we conducted a time course (namely, 30, 85, 100, 115, 125, 140, 155, and 170 days from gall initiation) analysis, where we measured both gall and leaf samples representing different developmental stages that spanned an entire growing season. To correlate these hormone data with developmental parameters during gall growth, we determined gall volume, tannin content, and aphid population size for the same time points. Interestingly, tannin production rose steeply in the early stages of gall development, while the aphid population size grew little. After this single peak (day 100), tannin concentrations declined moderately and aphid population size increased from then on. This switch in population growth was accompanied by notable changes in plant hormone titers. In general, all hormones but GA were elevated in all sample types isolated from the host tree (gall, leaves near and distant from gall) when compared with samples from an uninfected tree. Most of the elevated hormones showed similar changes over time; however, GA appeared to display the opposite behavior in all samples, suggesting that GA is a key target for controlling gall growth. When tannin concentrations spiked, GA levels peaked as well, while the remaining plant hormones exhibited a decline at that time. Principle component analysis revealed distinct functional groups in our hormone cohort. This yielded three groups comprising (1) CTK, ABA, ETH, and JA, (2) IAA and SA, (3) GA. The fact that GA comprised its own group and exhibited a unique profile during gall development prompted us to examine whether exogenous GA would alter the rate of gall growth. Indeed, we found that ectopic GA significantly accelerated gall growth, and more strongly than all other hormones, consistent with the notion that controlling GA levels within the gall is crucial for stimulating gall development. We propose a model, whereby the host plant downregulates GA concentrations in an attempt to throttle gall growth, while the gall-inducing aphid population counters these attempts.







Similar content being viewed by others
References
Allison SD, Schultz JC (2005) Biochemical responses of chestnut oak to a galling cynipid. J Chem Ecol 31:151–166
Chen JH (2000) Situation and prospect of chemical utilization of gall tannins in China. Chem Ind For Prod 20:70–82
Cooper W, Rieske L (2009) Woody stem galls interact with foliage to affect community associations. Environ Entomol 38:417–424
Cooper WR, Rieske LK (2010) Gall structure affects ecological associations of Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Environ Entomol 39:787–797
Cooper WR, Rieske LK (2011) Chestnut species and jasmonic acid treatment influence development and community interactions of galls produced by the Asian chestnut gall wasp, Dryocosmus kuriphilus. J Insect Sci 11:140
Cui K, He C, Zhang JG, Duan AG, Zeng YF (2012) Temporal and spatial profiling of Internode elongation-associated protein expression in rapidly growing culms of bamboo. J Proteome Res 11:2492–2507
Durbak A, Yao H, McSteen P (2012) Hormone signaling in plant development. Curr Opin Plant Biol 15:92–96
Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259
Gagné S, Lacampagne S, Claisse O, Gény L (2009) Leucoanthocyanidin reductase and anthocyanidin reductase gene expression and activity in flowers, young berries and skins of Vitis vinifera L. cv. Cabernet-Sauvignon during development. Plant Physiol Biochem 47:282–290
Guiscafré-Arrillaga J (1949) Formation of galls in stems and leaves of sugar cane in response to injections of growth-regulating substances. Phytopathology 39:489–493
Hartley S, Lawton J (1992) Host-plant manipulation by gall-insects: a test of the nutrition hypothesis. J Anim Ecol 61:113–119
Hou X, Ding L, Yu H (2013) Crosstalk between GA and JA signaling mediates plant growth and defense. Plant Cell Rep 32:1067–1074
Jiang Y, Joyce DC, Macnish AJ (2000) Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J Plant Growth Regul 19:106–111
Kawano T, Muto S (2000) Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. J Exp Bot 51:685–693
Khattab H, Khattab I (2005) Responses of Eucalypt trees to the insect feeding (Gall forming Psyllid). Int J Agric Biol 7:979–984
Lacampagne S, Gagné S, Gény L (2010) Involvement of abscisic acid in controlling the proanthocyanidin biosynthesis pathway in grape skin: new elements regarding the regulation of tannin composition and leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) activities and expression. J Plant Growth Regul 29:81–90
Lara I, Vendrell M (2000) Development of ethylene-synthesizing capacity in preclimacteric apples: interaction between abscisic acid and ethylene. J Am Soc Hortic Sci 125:505–512
Leatherdale D (1955) Plant hyperplasia induced with a cell-free insect extract. Nature 175:553–556
Mapes CC, Davies PJ (2001) Indole-3-acetic acid and ball gall development on Solidago altissima. New Phytol 151:195–202
McCalla D, Genthe MK, Hovanitz W (1962) Chemical nature of an insect gall growth-factor. Plant Physiol 37:98–103
Nabity PD, Haus MJ, Berenbaum MR, DeLucia EH (2013) Leaf-galling phylloxera on grapes reprograms host metabolism and morphology. Proc Natl Acad Sci USA 110:16663–16668
Nelson DR, Schuler MA, Paquette SM, Werck-Reichhart D, Bak S (2004) Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol 135:756–772
Ngakan OP, Yukawa J (1997) Synchronization with host plant phenology and gall site preference of Dinipponaphis autumna (Homoptera: Aphididae). Appl Entomol Zool 32:81–90
Price M, Van Scoyoc S, Butler L (1978) A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agric Food Chem 26:1214–1218
Price PW, Fernandes GW, Waring GL (1987) Adaptive nature of insect galls. Environ Entomol 16:15–24
Rehill B, Schultz J (2002) Opposing survivorship and fecundity effects of host phenology on the gall-forming aphid Hormaphis hamamelidis. Ecol Entomol 27:475–483
Santner A, Estelle M (2009) Recent advances and emerging trends in plant hormone signalling. Nature 459:1071–1078
Setha S (2012) Roles of abscisic acid in fruit ripening. Walailak J Sci Technol 9:297–308
Shao S, Yang Z, Chen X (2013) Gall development and clone dynamics of the galling aphid Schlechtendalia chinensis (Hemiptera: Pemphigidae). J Econ Entomol 106:1628–1637
Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16:2117–2127
Stone GN, Schönrogge K (2003) The adaptive significance of insect gall morphology. Trends Ecol Evol 18:512–522
Stone GN, Schönrogge K, Atkinson RJ, Bellido D, Pujade-Villar J (2002) The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu Rev Entomol 47:633–668
Straka JR, Hayward AR, Emery RN (2010) Gall-inducing Pachypsylla celtidis (Psyllidae) infiltrate hackberry trees with high concentrations of phytohormones. J Plant Interact 5:197–203
Sun TP (2011) The molecular mechanism and evolution of the GA–GID1–DELLA signaling module in plants. Curr Biol 21:R338–R345
Takei M, Yoshida S, Kawai T, Hasegawa M, Suzuki Y (2015) Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J Insect Physiol 72:43–51
Tanaka Y, Okada K, Asami T, Suzuki Y (2013) Phytohormones and willow gall induction by a gall-inducing sawfly. Biosci Biotechnol Biochem 77:1942–1948
Tokuda M (2012) Biology of Asphondyliini (Diptera: Cecidomyiidae). Entomol Sci 15:361–383
Tokuda M, Jikumaru Y, Matsukura K, Takebayashi Y, Kumashiro S, Matsumura M, Kamiya Y (2013) Phytohormones related to host plant manipulation by a gall-inducing leafhopper. PLoS One 8:e62350
Tooker JF, De Moraes CM (2011) Feeding by a gall-inducing caterpillar species alters levels of indole-3-acetic and abscisic acid in Solidago altissima (Asteraceae) stems. Arthropod-Plant Interact 5:115–124
Tooker JF, Rohr JR, Abrahamson WG, De Moraes CM (2008) Gall insects can avoid and alter indirect plant defenses. New Phytol 178:657–671
Tosaka Y, Nishida T (2007) Gall surface area is a simple and accurate measure of fitness in Nipponaphidini galling aphids (Homoptera: Aphididae). Appl Entomol Zool 42:217–221
Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19:2140–2155
Vierstra RD (2003) The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci 8:135–142
Wang C, Liu Y, Li S-S, Han G-Z (2015) Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiol 167:872–886
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot-Lond 100:681–697
Wool D (2004) Galling aphids: specialization, biological complexity, and variation. Annu Rev Entomol 49:175–192
Wool D, Bar-El N (1995) Population ecology of the galling aphid Forda formicaria von Heyden in Israel: abundance, demography, and gall structure. Israel J Zool 41:175–192
Wool D, Ben-Zvi O (1998) Population ecology and clone dynamics of the galling aphid Geoica wertheimae (Sternorrhyncha: Pemphigidae: Fordinae). Eur J Entomol 95:509–518
Wu B, Quilot B, Kervella J, Génard M, Li S (2003) Analysis of genotypic variation of sugar and acid contents in peaches and nectarines through the principle component analysis. Euphytica 132:375–384
Xie D-Y, Sharma SB, Paiva NL, Ferreira D, Dixon RA (2003) Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299:396–399
Yamaguchi H, Tanaka H, Hasegawa M, Tokuda M, Asami T, Suzuki Y (2012) Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol 196:586–595
Zhang M, Leng P, Zhang G, Li X (2009a) Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol 166:1241–1252
Zhang M, Yuan B, Leng P (2009b) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. U1402263, 31370651 and 31372266), the Grant for Essential Scientific Research of Chinese National Non-profit Institute (Grant No. CAFYBB2014ZD005), and the National High-tech R&D Program of China (Grant No. 2014AA021801).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E Kuzniak-Gebarowska.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2016_2224_MOESM1_ESM.tif
Figure S1. Exogenous phytohormone treatment during gall growth (n = 60). Controls received the same solution without corresponding phytohormone. Different letters in the same column represents differences at P ≤ 0.05 according to LSD (least significant difference) test. Bars represent SE. ABA: abscisic acid; CTK: cytokinin; DSGF: days since gall formation; GA: gibberellins; IAA: indole-3-acetic acid; STD: sodium tungstate dihydrate (TIFF 591 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Liu, J., Cui, K. et al. Gibberellic acid is selectively downregulated in response to aphid-induced gall formation. Acta Physiol Plant 38, 214 (2016). https://doi.org/10.1007/s11738-016-2224-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2224-5