Abstract
Objective
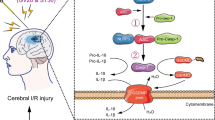
To investigate the effects of electroacupuncture (EA) at Neiguan (PC 6) and Baihui (GV 20) by observing the changes of CCAAT/enhancer-binding protein(C/EBP) homologous protein (CHOP) and caspase-12 gene expressions in rats after cerebral ischemia-reperfusion injury (IRI), and explore whether the apoptosis pathway of endoplasmic reticulum stress (ERS) is involved in the protective mechanisms of EA.
Methods
Sixty rats were randomly assigned to five groups (12 in each group): a normal control group (group A), a sham-operation group (group B), an operation group (group C), an Edaravone group (group D) and an EA group (group E). The cerebral IRI rat model was induced by middle cerebral artery occlusion (MCAO) using intraluminal monofilament. 2,3,5-triphenyl tetrazolium chloride (TTC) staining was adopted in the measurement of cerebral infarction volume. Real-time polymerase chain reaction (RT-PCR) was used to determine the mRNA expressions of CHOP and caspase-12.
Results
Compared with group A and group B, the volume of cerebral infarction and mRNA expressions of CHOP and caspase-12 in group C, group D and group E were increased, with statistical significances (P<0.05 or P<0.01); compared with group C, the volume of cerebral infarction and mRNA expressions of CHOP and caspase-12 in group D and group E were decreased significantly (P<0.05 or P<0.01); there were no significant differences between group D and group E in comparing the above items (P>0.05).
Conclusion
EA at Neiguan (PC 6) and Baihui (GV 20) can effectively suppress the volume of cerebral infarction. Furthermore, the underlying mechanism of EA at Neiguan (PC 6) and Baihui (GV 20) is possibly related to the down-regulation of CHOP and caspase-12 mRNA expressions, so as to decrease cell apoptosis.
摘要
目的
通过观察CHOP 和Caspase-12 基因表达在脑缺血再灌注大鼠体内的变化探讨电针内关、百会的效 应以及电针保护性机制是否与内质网应激介导的凋亡通路有关。
方法
将60 只大鼠随机分为5 组, 每组12 只, 即正常对照组(A)、假手术组(B)、模型组(C)、依达拉奉组(D)、电针干预组(E)。采用线栓法结扎大脑中动脉制备大 鼠局灶性脑缺血再灌注模型, 2,3,5-氯化三苯基四氮唑(TTC)染色测定脑梗死体积, RT-PCR 法测定缺血侧顶叶大脑皮 层CHOP、caspase-12 mRNA 表达变化。
结果
与正常组(A)和假手术组(B)比较, 模型组(C)、依达拉奉组(D)和电针 组(E)脑梗死体积百分比、CHOP、caspase-12 mRNA 表达均显著增高(P<0.01 或P<0.05); 与模型组(C)比较,依达拉 奉组(D)和电针组(E)脑梗死体积、CHOP、caspase-12 mRNA 表达均显著降低(P<0.01 或P<0.05);与依达拉奉组(D) 比较,电针组(E)脑梗死体积、CHOP、caspase-12 mRNA 表达无显著差异(P>0.05)。
结论
电针内关、百会可以有效 降低大鼠脑梗死体积,其脑保护潜在机制可能与下调凋亡因子CHOP、caspase-12 mRNA 表达, 从而抑制神经细胞 凋亡相关。
Similar content being viewed by others
References
Hossmann KA. Disturbances of cerebral protein synthesis and ischemic cell death. Brain Res, 1993, 96(1): 161–177.
De Gracia DJ, Kumar R, Owen C, Krause GS, White BC. Molecular pathways of protein synthesis inhibition during brain reperfusion: implications for neuronal survival or death. Cereb Blood Flow Metab, 2002, 22(2): 127–141.
Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res, 2010, 17(2): 189–202.
Lehotský J, Urban P, Pavlíková M, Tatarková Z, Kaminska B, Kaplán P. Molecular mechanisms leading to neuroprotection/ischemic tolerance: effect of preconditioning on the stress reaction of endoplasmic reticulum. Cell Mol Neurobiol, 2009, 29(6-7): 917–925.
Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominantnegative inhibitor of gene transcription. Genes Dev, 1992, 6(3): 439–453.
Barone MV, Crozat A, Tabaee A, Philipson L, Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev, 1994, 8(4): 453–464.
Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. Clin Invest, 2002, 109(4): 525–532.
Caspersen C, Pedersen PS, Treiman M. The sarco/ endoplasmic reticulum calcium-ATPase 2b is an endoplasmic reticulum stress-inducible prolein. J Biol Chem, 2000, 275(29): 22363–22372.
Paschen W.Disturbances of calcium homeostasis within the endoplasmic reticulum may contribute to the development of ischemic-cell damage. Med Hypotheses, 1996, 47(4): 283–288.
Pang L. To treat ischemic stroke with acupuncture by benefiting kidney and adjusting the Governor Vessel. Liaoning Zhongyi Zazhi, 2002, 29(8): 495–496.
Liu H. Review of 10 years’ clinical and pharmacological research on treating acute cerebral stroke through benefiting qi and improving blood circulation. Xiandai Zhongxiyi Jiehe Zazhi, 2000, 9(13): 217–219.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 1989, 20(1): 84–91.
Li ZR. Experimental Acupuncture Science. Beijing: China Press of Traditional Chinese Medicine, 2003: 327–329.
Ubeda M, Vallejo M, Habener JF. CHOP enhancement of gene transcription by interactions with Jun/Fos AP-l complex proteins. Mol Cell Biol, 1999, 19(11): 7589–7599.
Zhang DS. Piantan Fuzheng Decoction for 103 cases of acute cerebral ischemic infarction. Zhongyi Yanjiu, 1997, 10(4): 30.
Zhang PD. Observation of 78 patients with drowsiness through Shi’s Xing Nao Kai Qiao needling method. Zhongyi Waizhi Zazhi, 2006, 15(4): 60–61.
Shi XM. Awaking brain acupuncture for cerebral apoplexy. Zhongguo Linchuang Kangfu, 2003, 7(7): 1057–1058.
Shi XM, Zhao XF, Xiong J, Wen JR, Wang S. Clinical effect evaluation and proteomics research of awaking brain method for acute cerebral infarction. Tianjin Zhongyiyao, 2006, 23(5): 440.
Jiao YX, Zhang SZ. Clinical application of acupuncture at Neiguan (PC 6) to heart disease. Zhenjiu Linchuang Zazhi, 1997, 13(4): 75.
Du YB, Shi XM. Effect of acupuncture on ATP enzyme and cytochrome oxidase in acute cerebral ischemia in rats. Shanghai Zhenjiu Zazhi, 1999, 18(4): 38–39.
Du YB. Effect of acupuncture on microvascular wall ATP in acute cerebral infarction rats. Zhongguo Zhen Jiu, 2000, 20(10): 621.
Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res, 2005, 569(1-2): 29–63.
Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science, 2011, 333(6051): 1891–1894.
Oyadomari S, Mori M. Roles of CHOP/GADDl53 in endoplasmic reticulum stress. Cell Death Differ, 2004, 11(4): 381–389.
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol, 2001, 21(4): 1249–1259.
Oida Y, Shimazawa M, Imaizumi K, Hara H. Involvement of endoplasmic reticulum stress in the neuronal death included by transient forebrain ischemia in gerbil. Neuroscience, 2008, 151(2): 111–119.
Paschen W, Aufenberg C, Hotop S, Menqesdorf T. Transient cerebral ischemic activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. Cereb Blood Flow Metab, 2003, 23(4): 449–461.
Pasehen W, Gissel C, Linden T, Althausen S, Doutheil J. Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res Mol Brain Res, 1998, 60(1): 115–122.
Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ, 2004, 11(4): 403–415.
Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ, 2004, 11(4): 381–389.
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulumspecific apoptosis and cytotoxicity by amyloid-beta. Nature, 2000, 403(6765): 98–103.
Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience, 2003, 118(2): 491–499.
Mehmet H. Caspases find a new place to hide. Nature, 2000, 403(6765): 29–30.
Stull ND, Polan DP, Iacovitti L. Antioxidant compounds protect dopamine neurons from death due to oxidative stress in vitro. Brain Res, 2002, 931(2): 181–185.
Mizuno A, Umemura K, Nakashima M. Inhibitory effect of MCI-186, a free radical scavenger, on cerebral ischemia following rat middle cerebral artery occlusion. Gen Pharmaeol, 1998, 30(4): 575–578.
Nakashima M, Niwa M, Iwal T, Uematsu T. Involvement of free radicals in cerebral vascular reperfusion injury evaluated in a transient focal cerebral ischemia model of rat. Free Radic Biol Med, 1999, 26(5-6): 722–729.
Acknowledgments
This work was supported by National Natural Science Foundation of China (国家自然科学基金, No.81202771)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Wh., Yu, J., Lin, Yp. et al. Effect of electroacupuncture at Neiguan (PC 6) and Baihui (GV 20) on CHOP and caspase-12 gene expressions in rats after ischemia-reperfusion injury. J. Acupunct. Tuina. Sci. 15, 8–13 (2017). https://doi.org/10.1007/s11726-017-0967-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11726-017-0967-1
Keywords
- Acupuncture Therapy
- Electroacupuncture
- Point, Neiguan (PC 6)
- Point, Baihui (GV 20)
- Brain Ischemia
- Reperfusion Injury
- Apoptosis
- Rats