Abstract
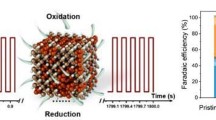
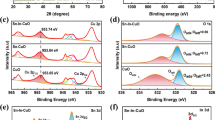
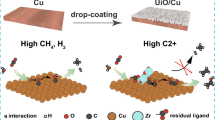
Electrocatalytic CO2 reduction (ECR) offers an attractive approach to realizing carbon neutrality and producing valuable chemicals and fuels using CO2 as the feedstock. However, the lack of cost-effective electrocatalysts with better performances has seriously hindered its application. Herein, a one-step co-electrodeposition method was used to introduce Zn, a metal with weak *CO binding energy, into Cu to form Cu/Zn intermetallic catalysts (Cu/Zn IMCs). It was shown that, using an H-cell, the high Faradaic efficiency of C2+ hydrocarbons/alcohols \(({\rm{F}}{{\rm{E}}_{{{\rm{C}}_{2 + }}}})\) could be achieved in ECR by adjusting the surface metal components and the applied potential. In suitable conditions, FEC2+ and current density could be as high as 75% and 40 mA/cm2, respectively. Compared with the Cu catalyst, the Cu/Zn IMCs have a lower interfacial charge transfer resistance and a larger electrochemically active surface area (ECSA), which accelerate the reaction. Moreover, the *CO formed on Zn sites can move to Cu sites due to its weak binding with *CO, and thus enhance the C–C coupling on the Cu surface to form C2+ products.
Similar content being viewed by others
References
He M, Sun Y, Han B. Green carbon science: Efficient carbon resource processing, utilization, and recycling towards carbon neutrality. Angewandte Chemie International Edition, 2022, 61(15): e202112835
Payra S, Shenoy S, Chakraborty C, et al. Structure-sensitive electrocatalytic reduction of CO2 to methanol over carbon-supported intermetallic PtZn nano-alloys. ACS Applied Materials & Interfaces, 2020, 12(17): 19402–19414
Pinthong P, Klongklaew P, Praserthdam P, et al. Effect of the nanostructured Zn/Cu electrocatalyst morphology on the electrochemical reduction of CO2 to value-added chemicals. Nanomaterials, 2021, 11(7): 1671–1682
Pori M, Arčon I, Lašič Jurković D, et al. Synthesis of a Cu/ZnO nanocomposite by electroless plating for the catalytic conversion of CO2 to methanol. Catalysis Letters, 2019, 149(5): 1427–1439
Wang X, Hu Q, Li G, et al. Recent advances and perspectives of electrochemical CO2 reduction toward C2+ products on Cu-based catalysts. Electrochemical Energy Reviews, 2022, 5(S2): 28–71
Keerthiga G, Chetty R. Electrochemical reduction of carbon dioxide on zinc-modified copper electrodes. Journal of the Electrochemical Society, 2017, 164(4): H164–H169
Sui P F, Gao M R, Liu S, et al. Carbon dioxide valorization via formate electrosynthesis in a wide potential window. Advanced Functional Materials, 2022, 32(32): 2203794–2203802
Wang L, Peng H, Lamaison S, et al. Bimetallic effects on Zn-Cu electrocatalysts enhance activity and selectivity for the conversion of CO2 to CO. Chem Catalysis, 2021, 1(3): 663–680
Yan Y, Ke L, Ding Y, et al. Recent advances in Cu-based catalysts for electroreduction of carbon dioxide. Materials Chemistry Frontiers, 2021, 5(6): 2668–2683
Wang W, Han J, Sun Y, et al. Metal-free SeBN ternary-doped porous carbon as efficient electrocatalysts for CO2 reduction reaction. ACS Applied Energy Materials, 2022, 5(9): 10518–10525
Tan X, Yu C, Ren Y, et al. Recent advances in innovative strategies for the CO2 electroreduction reaction. Energy & Environmental Science, 2021, 14(2): 765–780
Han J, Ma J, Zhou J, et al. Insight into the effect of surface coverage of carbon support on selective CO2 electroreduction to C2H4 over copper-based catalyst. Applied Surface Science, 2023, 609(30): 155394–155401
Wang J, Wang G, Zhang J, et al. Inversely tuning the CO2 electroreduction and hydrogen evolution activity on metal oxide via heteroatom doping. Angewandte Chemie International Edition, 2021, 60(14): 7602–7606
Kim D, Resasco J, Yu Y, et al. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nature Communications, 2014, 5(1): 4948
Zhu C, Kais S, Zeng X C, et al. Interfaces select specific stereochemical conformations: The isomerization of glyoxal at the liquid water interface. Journal of the American Chemical Society, 2017, 139(1): 27–30
Zhang Y, Wang X, Zheng S, et al. Hierarchical cross-linked carbon aerogels with transition metal-nitrogen sites for highly efficient industrial-level CO2 electroreduction. Advanced Functional Materials, 2021, 31(45): 2104377–2104386
Zhao Y, Zhang X G, Bodappa N, et al. Elucidating electrochemical CO2 reduction reaction processes on Cu(hkl) single-crystal surfaces by in situ Raman spectroscopy. Energy & Environmental Science, 2022, 15(9): 3968–3977
Mun Y, Lee S, Cho A, et al. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO. Applied Catalysis B: Environmental, 2019, 5(246): 82–88
Juntrapirom S, Santatiwongchai J, Watwiangkham A, et al. Tuning CuZn interfaces in metal–organic framework-derived electrocatalysts for enhancement of CO2 conversion to C2 products. Catalysis Science & Technology, 2021, 11(24): 8065–8078
Zhong X, Liang S, Yang T, et al. Sn dopants with synergistic oxygen vacancies boost CO2 electroreduction on CuO nanosheets to CO at low overpotential. ACS Nano, 2022, 16(11): 19210–19219
Zhu Z, Yu Z L, Gao W Y, et al. Controlled synthesis of intermetallic Au2Bi nanocrystals and Au2Bi/Bi heteronanocrystals with promoted electrocatalytic CO2 reduction properties. ChemSusChem, 2022, 15(10): 202200211
Kuang S, Li M, Chen X, et al. Intermetallic CuAu nanoalloy for stable electrochemical CO2 reduction. Chinese Chemical Letters, 2023, 34(7): 108013–108016
Jia S, Zhu Q, Chu M, et al. Hierarchical metal-polymer hybrids for enhanced CO2 electroreduction. Angewandte Chemie International Edition, 2021, 60(19): 10977–10982
Awais M, Kamal S, Ijaz F, et al. Improved catalytic performance of aspergillus flavus laccase immobilized on the zinc ferrite nanoparticles. Catalysis Letters, 2023, 5(153): 1240–1249
Yan S, Peng C, Yang C, et al. Electron localization and lattice strain induced by surface lithium doping enable ampere-level electrosynthesis of formate from CO2. Angewandte Chemie International Edition, 2021, 60(49): 25741–25745
Chen M, Wan S, Zhong L, et al. Dynamic restructuring of Cu-doped SnS2 nanoflowers for highly selective electrochemical CO2 reduction to formate. Angewandte Chemie International Edition, 2021, 60(50): 26233–26237
Nguyen D L T, Jee M S, Won D H, et al. Effect of halides on nanoporous Zn-based catalysts for highly efficient electroreduction of CO2 to CO. Catalysis Communications, 2018, 114(114): 109–113
Qin B, Li Y, Fu H, et al. Electrochemical reduction of CO2 into tunable syngas production by regulating the crystal facets of earth-abundant Zn catalyst. ACS Applied Materials & Interfaces, 2018, 10(24): 20530–20539
Leverett J, Tran-Phu T, Yuwono J A, et al. Tuning the coordination structure of Cu-N-C single atom catalysts for simultaneous electrochemical reduction of CO2 and NO3− to urea. Advanced Energy Materials, 2022, 12(32): 2201500–2201508
Sirisomboonchai S, Machida H, Bao Tran K V, et al. Efficient CO2 electrochemical reduction by a robust electrocatalyst fabricated by electrodeposition of indium and zinc over copper foam. ACS Applied Energy Materials, 2022, 5(8): 9846–9857
Xu A, He B, Yu H, et al. A facile solution to mature cathode modified by hydrophobic dimethyl silicon oil (DMS) layer for electro-Fenton processes: Water proof and enhanced oxygen transport. Electrochimica Acta, 2019, 10(308): 158–166
Feng Y, Yang H, Zhang Y, et al. Te-doped Pd nanocrystal for electrochemical urea production by efficiently coupling carbon dioxide reduction with nitrite reduction. Nano Letters, 2020, 20(11): 8282–8289
Chen D, Zhou Z, Feng C, et al. An upgraded lithium ion battery based on a polymeric separator incorporated with anode active materials. Advanced Energy Materials, 2019, 9(15): 1803627–1803637
Li L, Jin X, Yu X, et al. Bimetallic Cu-Bi catalysts for efficient electroreduction of CO2 to formate. Frontiers in Chemistry, 2022, 10(10): 983778
Gao Y, Yu S, Zhou P, et al. Promoting electrocatalytic reduction of CO2 to C2H4 production by inhibiting C2H5OH desorption from Cu2O/C composite. Small, 2022, 18(9): 2105212
Shan W, Liu R, Zhao H, et al. In situ surface-enhanced Raman spectroscopic evidence on the origin of selectivity in CO2 electrocatalytic reduction. ACS Nano, 2020, 14(9): 11363–11372
Hoang T T H, Verma S, Ma S, et al. Nanoporous copper-silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. Journal of the American Chemical Society, 2018, 140(17): 5791–5797
Hu M, Cai Z, Yang S, et al. Direct growth of uniform bimetallic core-shell or intermetallic nanoparticles on carbon via a surface-confinement strategy for electrochemical hydrogen evolution reaction. Advanced Functional Materials, 2023, 33(13): 2212097–2212106
Ma Y, Yu J, Sun M, et al. Confined growth of silver-copper Janus nanostructures with 100 facets for highly selective tandem electrocatalytic carbon dioxide reduction. Advanced Materials, 2022, 34(19): 2110607
Yan T, Wang P, Xu Z H, et al. Copper (II) frameworks with varied active site distribution for modulating selectivity of carbon dioxide electroreduction. ACS Applied Materials & Interfaces, 2022, 14(11): 13645–13652
Sang J, Wei P, Liu T, et al. A reconstructed Cu2P2O7 catalyst for selective CO2 electroreduction to multicarbon products. Angewandte Chemie International Edition, 2022, 61(5): e202114238
Xu J, Sun Y, Lu M, et al. One-step electrodeposition fabrication of Ni3S2 nanosheet arrays on Ni foam as an advanced electrode for asymmetric supercapacitors. Science China Materials, 2019, 62(5): 699–710
Grosse P, Yoon A, Rettenmaier C, et al. Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nature Communications, 2021, 12(1): 6736–6746
Kunze S, Tanase L C, Prieto M J, et al. Plasma-assisted oxidation of Cu(100) and Cu(111). Chemical Science, 2021, 12(42): 14241–14253
Acknowledgements
The work was supported by the National Key R&D Program of China (Grant No. 2020YFA0710201), the China Postdoctoral Science Foundation (Grant No. 2023M731096), the National Natural Science Foundation of China (Grant Nos. 22022307, 22121002, and 21890761), and the Research Funds of Happiness Flower ECNU (Grant No. 2020ST2203).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Deng, T., Jia, S., Han, S. et al. Electrochemical CO2 reduction to C2+ products over Cu/Zn intermetallic catalysts synthesized by electrodeposition. Front. Energy 18, 80–88 (2024). https://doi.org/10.1007/s11708-023-0898-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11708-023-0898-0