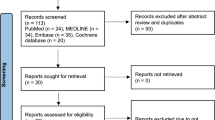
Abstract
Robot assisted surgery (RAS) is increasingly used, and besides conventional minimally invasive surgery (cMIS) surgeons are challenged to learn an increased array of skills. This study aimed to assess the influence of both learning curves on each other. A prospective randomized crossover study was performed. Participants without cMIS or RAS experience (Groups 1 and 2), and cMIS experienced, (Group 3) were recruited. Three suturing tasks (intracorporal suturing, tilted plane and anastomosis needle transfer) were performed on the EoSim cMIS simulator or RobotiX RAS simulator up to twenty repetitions. Subsequently, Groups 1 and 2 performed the tasks on the other modality. Outcomes were simulator parameters, validated composite and pass/fail scores. In total forty-three participants were recruited. Overall RAS suturing was better in Group 1 (cMIS followed by RAS tasks) and 3 (RAS tasks) versus Group 2 (RAS followed by cMIS tasks) for time (163 s and 157 s versus 193 s p = 0.004, p = 0.001) and composite scores (92/100 and 91/100 versus 89/100 p = 0.008, p = 0.020). The cMIS suturing was better for Group 2 versus 1 (time 287 s versus 349 s p = 0.005, composite score 96/100 versus 94/100 p = 0.002). Significant differences from the RAS suturing pass/fail were reached earlier by Group 3, followed by Groups 1 and 2 (repetition six, nine and twelve). In cMIS suturing Group 2 reached significant differences from the pass/fail earlier than Group 1 (repetition four versus six). Transferability of skills was shown for cMIS and RAS, indicating that suturing experience on cMIS or RAS is beneficial in learning either approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After the introduction of robot-assisted surgery (RAS) with the DaVinci system (Intuitive Surgical Inc. USA), the frequency and application of the usage of RAS has been growing continuously [1]. With the field of general surgery as the most recent growing surgical specialism, residents in training and specialized surgeons, which are often highly skilled in conventional minimally invasive surgery (cMIS), are being challenged to attain and maintain technical skills in both methods [2]. This is especially the case for residents in training who are novices and need to acquire proficiency in both techniques. Additionally, despite the increasing implementation of RAS in clinical practice, operative participation of residents is limited, because a dual console is lacking frequently [3]. This leads to an increasing demand for validated and standardized RAS curricula and expresses the importance of simulation training to acquire competency outside the clinical setting [4,5,6,7]. Skills acquisition of RAS simulation training has been shown to be faster compared to cMIS simulation training for novices [8,9,10]. There is still discussion on the level of skill transfer between both modalities, although there are similarities between RAS and cMIS. There are several studies performed which have found a transfer effect from cMIS to RAS [2, 11,12,13,14,15,16,17]. However, the transferability effect of RAS on cMIS is more unclear with three studies showing no effect and two reporting a positive transfer effect [11, 13, 15,16,17]. Most of these studies concerned basic peg transfer skills [12,13,14,15,16] or studied only the initial part of a learning curve with limited number of repetitions [2, 11, 12, 14, 17], whereas the main benefit of RAS is expected during the performance of complex skills such as suturing. Therefore, this study aims to assess the skills transferability of cMIS and RAS during a suturing learning curve in a crossover simulated setting. With this study we aim to give further insight in complex skill acquisition to improve competence-based training for surgical residents.
Methods
Participants
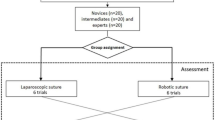
Participants were recruited voluntary at Radboud university medical centre Nijmegen, The Netherlands. To include participants with a basic level of surgical understanding of the concept of cMIS and RAS but without experience in these techniques, medical interns and physician researchers were recruited for either Group 1 or 2. After recruitment, allocation was based randomly on the basis of the current simulator availability without stratification. To include participants experienced with cMIS but inexperienced with RAS, surgical residents and surgeons were included in Group 3. Due to the non-medical setup and voluntary participation of this study, no ethical board approval was required.
Simulators
EoSim cMIS AR simulator
To compare instrument parametric outcomes of both modalities the EoSim minimally invasive surgical augmented reality (AR) simulator (Eosurgical ltd., Edinburgh, Scotland, UK) was used in this study (Fig. 1). Previous validation studies reported evidence of multiple aspects of validity for this simulator [18,19,20,21,22]. The EoSim setup consisted of a suitcase model the participants performance displayed on a connected laptop with the provided Surgtrac software. To assure correct positioning of the screen an adjustable standard was used. The simulator parametric outcomes consisted of the parameters; total time, distance, working space and off-screen (Table 1). Additionally, a researcher noted the number of sutures made during the third task to determine the time per suture and instrument distance per suture.
RobotiX RAS VR simulator
For the robot-assisted tasks the RobotiX virtual reality (VR) simulator was used (Fig. 1) which showed evidence for validity in previous studies on multiple aspects [23,24,25,26,27,28,29,30]. The system, consists of a main console for a trainee and a control tower, 3D Systems (3D Systems Inc., Cleveland OH). Participants were able to adjust working height and eye level of the main console. The ‘Mentorlearn’ software kept track of each individual performance and progress of the custom-constructed learning curve curriculum used for this study. The software parametric outcomes consisted of multiple parameters which were divided into movement, safety and task-specific parameters as shown in Table 1.
Tasks
To simulate the required skills during complex suturing the tasks for this study were selected to represent the separate components. Therefore, Task 1 consisted of an intracorporal suture and knot tying task, Task 2 consisted of a tilted plane needle transfer and Task 3 of an anastomosis needle transfer task. The cMIS Tasks were previously developed and validated [20]. Further specification of the RAS and cMIS tasks are described in the supplementals of a previously performed learning curve study [8]. The third task was selected to support the skill acquisition of Tasks 1 and 2. Therefore, only a limited amount of repetitions (each repetition consisting of eight to ten needle transfers) were performed.
Protocol
After recruitment, all participants completed a questionnaire regarding their informed consent, demographic information and surgical experience. Depending on the available simulator participants received an instruction and subsequently performed two familiarization tasks on either the EoSim or the RobotiX as shown in Fig. 2. Participants in Group 1 started with tasks on the EoSim followed by the RobotiX simulator and participants in Group 2 started with the RobotiX followed by the EoSim simulator. Participants in Group 3 only performed tasks on the RobotiX simulator. This group was included to assess the difference between clinical cMIS experience and simulator cMIS experience. After accomplishing familiarization tasks, the repetitive training of the suturing tasks was performed under supervision of a researcher up to a maximum of one hour and divided in multiple sessions. The researcher kept track of each participants progress and data storage. The intracorporal suturing (Task 1) training was regarded complete after twenty repetitions or three consecutive similar performances in a row based on the time parameter. The number of repetitions was based on a previous study by Botden et al. showing a suturing learning curve in fifteen repetitions on a laparoscopic simulator [31]. Therefore, twenty repetitions were used to achieve a substantial part of the learning curve. Due to the high visual and parametric similarity on both systems Task 1 was used as the ‘main task’ for learning curve evaluation. Task 2 was completed for the cMIS learning curve after fifteen repetitions and for RAS after three repetitions (one repetition consists of five suture transfers). Task 3 was regarded complete after three repetitions on both the EoSim and RobotiX simulator. After completion of the required repetitions on either cMIS or RAS the participant switched to the other modality, where they performed the suturing tasks after the instruction and introduction tasks. The main outcome of this study were the calculated composite score for each task. Secondary outcomes were the time, safety and movement parameters.
Statistical analysis
The statistical analysis was performed using Statistical Package for Social Sciences (SPSS) software version 25 (IBM Corp., Armonk NY). All p-values of < 0.05 were considered statistically significant. Based on a previous RAS validation study the power analysis resulted in a minimal of eight participants per group to demonstrate a difference RAS composite score of fourteen points (Means 87 versus 73, Standard deviation 10, β = 0.2 and α = 0.05) [32]. Composite scores for the cMIS tasks were calculated using data from a previous study where construct validity was shown for several parameters per task (Table 1) [20]. The cMIS composite scores were calculated after normalization of the parameters showing construct and range from 0 to 100 with 100 being the best score. Accordingly, a cut-off score was calculated using the contrasting groups analysis model from the study by Jørgenson et al. [33]. This analysis calculates the optimal cut-off value (lowest false positive and false negative ratio) between novice and expert users. For the RAS tasks the composite scores were based on the validated parameters shown in Table 1 [32]. Additionally, the suturing composite scores were compared to the calculated cut-off value from the contrasting groups analysis using a one-sample t-test. Differences between two groups were calculated using the independent t-test and a one-way ANOVA was used when comparing three groups.
Results
Demographics
A total of 43 participants completed this study as shown in Table 2. Group 1 (n = 16) and Group 2 (n = 10) consisted of medical students and physician researchers with a mean age of 23 and 24 years, respectively. The laparoscopic experienced group (Group 3, n = 17) consisted of thirteen residents in training and four surgeons. The specialties in group 3 ranged from abdominal/oncological surgery to urology, gynaecology and paediatric surgery participants. The difference in group size was due to the difference in availability of the simulators as participants in Group 3 only performed the RAS simulation tasks. The total minutes Group 1 and Group 2 spent of the performance of all three task showed a minor difference of 19 min (p = 0.514).
Learning curve progression
The learning curve progression was based on the intracorporeal suturing task (Task 1). During the cMIS learning curve, participants performed at least sixteen suture repetitions in Group 1 and eighteen in Group 2. The complete twenty repetitions were performed by nine participants in Group 1 and seven in Group 2. The twenty suturing repetitions during the RAS learning curve was completed by twelve participants in Group 1, eight in Group 2 and ten in Group 3. The participants completed at least eighteen repetitions in Group 1 and nineteen repetitions in Group 2 and Group 3. During cMIS Task 2 at least ten repetitions were completed by Group 1 and twelve by Group 2. The complete fifteen repetitions were accomplished by twelve participants in Group 1 and seven in Group 2. The three repetitions required to complete RAS Task 2 and 3 learning curves were achieved by all participants in Group 2 and Group 3. Due to limited time for training one participant in Group 1 was unable to perform the third repetition of RAS Task 2 and 3 but was included in the analysis. The cMIS Task 3 was completed by all participants.
Effects cMIS training on RAS performance
Overall
The RAS intracorporal suturing task resulted in a beneficial effect in a composite score and task time from the cMIS simulator (Group 1) or previous clinical laparoscopic experience (Group 3) as shown in Table 3 and Fig. 3. Comparing Group 2 to Groups 1 and 3, a significantly longer task time (p = 0.004 and p = 0.001) and a lower composite score was found (p = 0.008 and v0.020). The participants in Group 3 only showed a statistically significant faster performance time for Task 3 versus Group 1 (314 s versus 393 s, p = 0.021). No significant beneficial effect in task time or composite score was shown between participants in Group 2 versus Groups 1 or 3.
RAS intracorporeal suturing
The RAS suturing task appeared to be completed in less time and with a higher composite score by participants in Group 1 compared to Group 2 (Fig. 4 and 5). However, statistically significant differences between Groups 1 and 2 were only evident for task time at repetition sixteen (113 s versus 155 s, p = 0.030) and for the composite score at repetition three (89 versus 78, p = 0.017, see Appendix 1). Group 3 showed similar performance as Group 1 and was statistically different in task time at repetition sixteen (93 versus 91, p = 0.013). A significant difference from the cut-off value was reached first by Group 3 at repetition six, then by Group 1 at repetition nine and finally by Group 2 at repetition twelve. The needle handling was on average scored better by Group 1 with less needle drops compared to Group 2, however, without any statistically significant difference. The needle was held significantly more off-screen by Group 1 versus Group 2 during repetition nine (5.1 s versus 0.6 s, p = 0.016), eleven (3.6 s versus 0.4 s, p = 0.047) and seventeen (0.8 s versus 0.04 s, p = 0.026).
RAS tilted plane and anastomosis needle transfer
Overall results of Task 2 and 3 are shown in Table 3. The tilted plane needle transfer (Task 2) was completed by all groups with similar results on a composite score and time resulting in no significant differences. The anastomosis needle transfer (Task 3) was performed best by the participants with cMIS experience (Group 3) with the highest composite score and the lowest time results. However, only the time parameter showed a statistically significantly difference compared to Group 1 (p = 0.021). with an overall time difference of 79s.
Effects RAS training on cMIS performance
Overall
Performance results from the overall scores are shown in Table 3. During the cMIS intracorporal suturing task (Task 1) a possible beneficial effect of RAS simulator experience is shown for Group 2 with an overall statistically significant higher composite score of 2 points (p = 0.002) and 62 s faster task time (p = 0.005). Similar results for the overall outcomes are shown for Task 2 and 3. Group 2 shows a mean faster performance for Tasks 2 and 3 and a higher mean composite score at Task 3, although not statistically significant.
cMIS intracorporeal suturing
The suturing task was performed by Group 2 with a higher composite score and less time during the first ten repetitions (Fig. 3 and 5). Statistically this was shown to be significantly different in composite score compared to Group 1 for repetitions five (79 versus 88, p = 0.023), six (85 versus 91, p = 0.024), seven (85 versus 90, p = 0.046) and nine (88 versus 94, p = 0.025), see Appendix 1. In terms of task time Group 2 also showed statistically significantly better performance for the repetitions five (477 s versus 323 s, p = 0.036), six (380 s versus 278 s, p = 0.023), nine (309 s versus 180 s, p = 0.010) and fifteen (226 s versus 167 s, p = 0.034). Additionally, Group 2 showed an earlier significant difference from the cut-off value by two repetitions (Repetition 4 versus 6). The distance and working space parameters were performed with a steeper curve by Group 2 in the first half of the learning curve with multiple statistically significantly different repetitions. The participants in Group 2 reached early a stable plateau curve on the off-screen parameter compared to Group 1 with seven repetitions showing a statistically significant difference from which five range between repetition 12 and 20.
cMIS tilted plane and anastomosis needle transfer
Task 2 was performed equally overall by both groups shown in Table 3. The anastomosis needle transfer (Task 3) however, was performed with higher outcomes by Group 2 with a higher composite score and shorter time results. Although, no statistically significant difference could be found between groups.
Discussion
This study shows a transferability effect from cMIS to RAS and vice versa. During cMIS training the RAS experienced participants showed a faster composite score differentiation from the pass/fail value, an initial steeper decrease in time and a better off-screen performance. This indicates that RAS experience leads to faster competency and safer performance during cMIS suturing tasks. The participants with cMIS training showed an earlier significant difference from the pass/fail value and were significantly better in the overall time and composite score results compared to RAS novices. However, cMIS experience showed a negative effect on the off-screen performance during RAS training, which emphasizes this as a critical parameter to be monitored during RAS training. The clinical cMIS experience was shown to distinguish earlier from the RAS pass/fail score compared to cMIS training experience but did not show other major differences in the performance outcomes.
The results of this study support previous findings regarding the transferability of skills from cMIS to RAS [2, 11, 12, 14,15,16,17], although these mainly focussed on basic skills. The study by Thomaier et al. showed a beneficial effect of cMIS or RAS training of a peg transfer task on both cMIS and RAS performance by novices [15]. Interestingly, the results of Thomaier et al. also found that after cMIS training there was no improvement in the off-screen parameter between RAS baseline and post-training performance. Whereas, the RAS training group showed a better improvement in the instruments off-screen parameter. These results indicate the importance of the off-screen parameter to be monitored for RAS trainees with cMIS experience as the lack of haptic feedback and off-screen instruments could cause unwanted tissue damage.
The skill transfer of RAS to cMIS was previously not found [11, 13, 16]. Whereas, the studies by Thomaier and Obek et al. did report a positive transfer effect from RAS to cMIS [15, 17]. These discrepancies are likely to be explained by the limited amount of repetitions or tasks used. This study showed the most positive skill transfer of RAS to cMIS, indicating that the more “intuitive” setting of RAS could be used for novice trainees to improve the initial difficulty of the cMIS learning curve. Our study is the first to examine a learning curve of multiple suturing tasks for up to twenty repetitions. Additionally, this study is strengthened by the randomized crossover setup and includes a separate group of participants with clinical cMIS experience. As stated by Kassite et al. the assessment of a learning curve should be based on multiple parameters [34]. In agreement with this statement, this study included multiple parameters, used in the composite scores and separately.
However, this study also has some limitations. At our institution, there was no availability of a DaVinci system for training purposes. This limited the possibility of matching the suturing tasks in detail on both modalities. However, the main goal of this study was not to compare the cMIS versus the RAS skill improvement but to assess the transferability of skills for which this setup worked well. Another limitation is the difference in group size Group 1 (n = 16) and Group 2 (n = 10) which was based on the simulator availability during training times. However, Group 3 (n = 17) was only trained on the RAS simulator and, therefore, more participants were included according to protocol in the cMIS first group.
Conclusion
The results of this study showed a transferability effect of skills from cMIS to RAS and from RAS to cMIS. The experience in either cMIS or RAS resulted in a faster differentiation from the used pass/fail score on the contrasting modality. Additionally, the RAS experience had a positive effect on the off-screen performance and could be beneficial for the learning of safer cMIS performances. These results indicate that previous cMIS or RAS experience shortens the learning curve and a mixed approach of RAS and cMIS training could be used when learning either of the modalities.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Data are located in controlled access data storage at the Radboud University Medical Centre.
References
Intuitive Surgical Annual Report. (2018) Intuitive Surgical Incorporated. http://www.annualreports.com/Company/intuitive-surgical-inc. Accessed 28–10–2019
Chandra V, Nehra D, Parent R, Woo R, Reyes R, Hernandez-Boussard T, Dutta S (2010) A comparison of laparoscopic and robotic assisted suturing performance by experts and novices. Surgery 147(6):830–839. https://doi.org/10.1016/j.surg.2009.11.002
Vetter MH, Palettas M, Hade E, Fowler J, Salani R (2018) Time to consider integration of a formal robotic-assisted surgical training program into obstetrics/gynecology residency curricula. J Robot Surg 12(3):517–521. https://doi.org/10.1007/s11701-017-0775-0
Zhao B, Lam J, Hollandsworth HM, Lee AM, Lopez NE, Abbadessa B, Eisenstein S, Cosman BC, Ramamoorthy SL, Parry LA (2019) General surgery training in the era of robotic surgery: a qualitative analysis of perceptions from resident and attending surgeons. Surg Endosc. https://doi.org/10.1007/s00464-00019-06954-00460.10.1007/s00464-019-06954-0
Carpenter BT, Sundaram CP (2017) Training the next generation of surgeons in robotic surgery. Robot Surg 4:39–44. https://doi.org/10.2147/RSRR.S70552
Chen R, Rodrigues Armijo P, Krause C, Force SRT, Siu K-C, Oleynikov D (2019) A comprehensive review of robotic surgery curriculum and training for residents, fellows, and postgraduate surgical education. Surg Endosc. https://doi.org/10.1007/s00464-00019-06775-00461.10.1007/s00464-019-06775-1
MacCraith E, Forde JC, Davis NF (2019) Robotic simulation training for urological trainees: a comprehensive review on cost, merits and challenges. J Robot Surg 13(3):371–377. https://doi.org/10.1007/s11701-019-00934-1
Leijte E, de Blaauw I, Van Workum F, Rosman C, Botden S (2019) Robot assisted versus laparoscopic suturing learning curve in a simulated setting. Surg Endosc. https://doi.org/10.1007/s00464-00019-07263-00462.10.1007/s00464-019-07263-2
Marecik SJ, Chaudhry V, Jan A, Pearl RK, Park JJ, Prasad LM (2007) A comparison of robotic, laparoscopic, and hand-sewn intestinal sutured anastomoses performed by residents. Am J Surg 193(3):349–355. https://doi.org/10.1016/j.amjsurg.2006.09.018
Passerotti CC, Franco F, Bissoli JCC, Tiseo B, Oliveira CM, Buchalla CAO, Inoue GNC, Sencan A, Sencan A, do Pardo RR, Nguyen HT, (2015) Comparison of the learning curves and frustration level in performing laparoscopic and robotic training skills by experts and novices. Int Urol Nephrol 47(7):1075–1084. https://doi.org/10.1007/s11255-015-0991-3
Anderberg M, Larsson J, Kockum CC, Arnbjornsson E (2010) Robotics versus laparoscopy–an experimental study of the transfer effect in maiden users. Ann Surg Innov Res 4:3. https://doi.org/10.1186/1750-1164-4-3
Davila DG, Helm MC, Frelich MJ, Gould JC, Goldblatt MI (2018) Robotic skills can be aided by laparoscopic training. Surg Endosc 32(6):2683–2688. https://doi.org/10.1007/s00464-017-5963-5
Hassan SO, Dudhia J, Syed LH, Patel K, Farshidpour M, Cunningham SC, Kowdley GC (2015) Conventional Laparoscopic vs Robotic Training: Which is Better for Naive Users? A Randomized Prospective Crossover Study. J Surg Educ 72(4):592–599. https://doi.org/10.1016/j.jsurg.2014.12.008
Panait L, Shetty S, Shewokis PA, Sanchez JA (2014) Do laparoscopic skills transfer to robotic surgery? J Surg Res 187(1):53–58. https://doi.org/10.1016/j.jss.2013.10.014
Thomaier L, Orlando M, Abernethy M, Paka C, Chen CCG (2017) Laparoscopic and robotic skills are transferable in a simulation setting: a randomized controlled trial. Surg Endosc 31(8):3279–3285. https://doi.org/10.1007/s00464-016-5359-y
Moncayo S, Compagnon R, Caire F, Grosos C, Bahans C, Ilhero P, Fourcade L, Ballouhey Q (2019) Transition effects from laparocscopic to robotic surgery skills in small cavities. J Robot Surg. https://doi.org/10.1007/s11701-11019-01024-y.10.1007/s11701-019-01024-y
Obek C, Hubka M, Porter M, Chang L, Porter JR (2005) Robotic versus conventional laparoscopic skill acquisition: implications for training. J Endourol 19(9):1098–1103. https://doi.org/10.1089/end.2005.19.1098
Arts EEA, Leijte E, Witteman BPL, Jakimowicz JJ, Verhoeven B, Botden SMBI (2019) Face, Content, and Construct Validity of the Take-Home EoSim Augmented Reality Laparoscopy Simulator for Basic Laparoscopic Tasks. J Laparoendosc Adv Surg Tech A 29(11):1419–1426. https://doi.org/10.1089/lap.2019.0070
Hennessey IAM, Hewett P (2013) Construct, concurrent, and content validity of the eoSim laparoscopic simulator. J Laparoendosc Adv Surg Tech A 23(10):855–860. https://doi.org/10.1089/lap.2013.0229
Leijte E, Arts E, Witteman B, Jakimowicz J, De Blaauw I, Botden S (2019) Construct, content and face validity of the eoSim laparoscopic simulator on advanced suturing tasks. Surg Endosc 33(11):3635–3643. https://doi.org/10.1007/s00464-018-06652-3
Mansoor SM, Våpenstad C, Mårvik R, Glomsaker T, Bliksøen M (2019) Construct validity of eoSim - a low-cost and portable laparoscopic simulator. Minim Invasive Ther Allied Technol:1–8. doi:https://doi.org/10.1080/13645706.2019.1638411
Retrosi G, Cundy T, Haddad M, Clarke S (2015) Motion Analysis-Based Skills Training and Assessment in Pediatric Laparoscopy: Construct, Concurrent, and Content Validity for the eoSim Simulator. J Laparoendosc Adv Surg Tech A 25(11):944–950. https://doi.org/10.1089/lap.2015.0069
Amirian MJ, Lindner SM, Trabulsi EJ, Lallas CD (2014) Surgical suturing training with virtual reality simulation versus dry lab practice: an evaluation of performance improvement, content, and face validity. J Robot Surg 8(4):329–335. https://doi.org/10.1007/s11701-014-0475-y
Harrison P, Raison N, Abe T, Watkinson W, Dar F, Challacombe B, Van Der Poel H, Khan MS, Dasgupa P, Ahmed K (2018) The Validation of a Novel Robot-Assisted Radical Prostatectomy Virtual Reality Module. J Surg Educ 75(3):758–766. https://doi.org/10.1016/j.jsurg.2017.09.005
Hertz AM, George EI, Vaccaro CM, Brand TC (2018) Head-to-Head Comparison of Three Virtual-Reality Robotic Surgery Simulators. JSLS : Journal of the Society of Laparoendoscopic Surgeons 22 (1):e2017.00081. doi:https://doi.org/10.4293/JSLS.2017.00081
Hovgaard LH, Andersen SAW, Konge L, Dalsgaard T, Larsen CR (2018) Validity evidence for procedural competency in virtual reality robotic simulation, establishing a credible pass/fail standard for the vaginal cuff closure procedure. Surg Endosc 32(10):4200–4208. https://doi.org/10.1007/s00464-018-6165-5
Omar I, Dilley J, Pucher P, Pratt P, Ameen T, Vale J, Darzi A, Mayer E (2017) The RobotiX simulator: face and content validation using the fundamentals of robotic surgery (FRS) curriculum. J Urol 197(4):e700–e701. https://doi.org/10.1016/j.juro.2017.02.1626
Watkinson W, Raison N, Abe T, Harrison P, Khan S, Van der Poel H, Dasgupta P, Ahmed K (2018) Establishing objective benchmarks in robotic virtual reality simulation at the level of a competent surgeon using the RobotiX Mentor simulator. Postgrad Med J 94(1111):270–277. https://doi.org/10.1136/postgradmedj-2017-135351
Whittaker G, Aydin A, Raison N, Kum F, Challacombe B, Khan MS, Dasgupta P, Ahmed K (2016) Validation of the RobotiX Mentor Robotic Surgery Simulator. J Endourol 30(3):338–346. https://doi.org/10.1089/end.2015.0620
Whittaker G, Aydin A, Raveendran S, Dar F, Dasgupta P, Ahmed K (2019) Validity assessment of a simulation module for robot-assisted thoracic lobectomy. Asian Cardiovasc Thorac Ann 27(1):23–29. https://doi.org/10.1177/0218492318813457
Botden SMBI, de Hingh IHJT, Jakimowicz JJ (2009) Suturing training in Augmented Reality: gaining proficiency in suturing skills faster. Surg Endosc 23(9):2131–2137. https://doi.org/10.1007/s00464-008-0240-2
Leijte E, De Blaauw I, Rosman C, Botden S (2020) Validation of the RobotiX robot assisted surgery simulator on advanced suturing tasks. Manuscript submitted for publication
Jørgensen M, Konge L, Subhi Y (2018) Contrasting groups’ standard setting for consequences analysis in validity studies: reporting considerations. Adv Simul (Lond) 3:5–5. https://doi.org/10.1186/s41077-018-0064-7
Kassite I, Bejan-Angoulvant T, Lardy H, Binet A (2019) A systematic review of the learning curve in robotic surgery: range and heterogeneity. Surg Endosc 33(2):353–365. https://doi.org/10.1007/s00464-018-6473-9
Acknowledgements
We would like to thank EoSurgical and Simbionix (3D Systems) for making their simulators available for this study.
Funding
Erik Leijte, Ivo De Blaauw, Camiel Rosman and Sanne Botden have no conflict of interest or financial ties to disclose, no funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by EL and SB. The first draft of the manuscript was written by EL. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leijte, E., De Blaauw, I., Rosman, C. et al. Transferability of the robot assisted and laparoscopic suturing learning curves. J Robotic Surg 18, 56 (2024). https://doi.org/10.1007/s11701-023-01753-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11701-023-01753-1