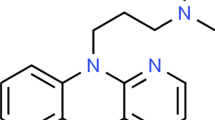
Abstract
The kinetics of the oxidation reaction of Hydroxyzine hydrochloride with potassium peroxymonosulfate was studied depending on the pH of the medium. It was established for the first time that the kinetics of the reaction of N-oxidation of Hydroxyzine obeyed the general laws of the mechanism of specific acid–base catalysis, proceeded quantitatively and stoichiometrically according to the mechanism of nucleophilic substitution of the β-oxygen atom of the peroxyacid group of peroxymonosulfate ions. It was shown that quantitative oxidation was achieved in 1–1.5 min at pH 8.0–8.5. The only reaction product was Hydroxyzine N-oxide. A scheme of the oxidation process was proposed. Techniques were developed and the possibility of quantitative determination of Hydroxyzine hydrochloride in the substance and tablets of 25 mg by iodometry using Oxone as an analytical reagent was shown. A known excess of reagent was added, and after a predetermined time, the residual reagent was determined iodometrically. RSD ≤ 1.52%. (\(\overline{x} - \mu\))·100/μ < RSD. The procedures had several advantages such as speed, simplicity, accuracy, selectivity, and cost-effectiveness, and therefore could be easily adapted by quality control laboratories for routine analysis.






Similar content being viewed by others
References
Altamura AC, Moliterno D, Paletta S, Maffini M, Mauri MC, Bareggi S (2013) Understanding the pharmacokinetics of anxiolytic drugs. Expert Opin Drug Metab Toxicol 9(4):423–440. https://doi.org/10.1517/17425255.2013.759209
Anwar A (2012) Wassel characteristic of membrane sensors for the selective determination of some antihistaminic pharmaceutical formulations. Anal Bioanal Electrochem 4(1):17–31
Basavaiah K, Charan VS (2002) Charan Titrimetric and spectrophotometric assay of some antihistamines through the determination of the chloride of their hydrochlorides. Il Farmaco 57(1):9–17. https://doi.org/10.1016/S0014-827X(01)01151-X
Beltagi AM, Abdallah OM, Ghoneim MM (2008) Development of a voltammetric procedure for assay of the antihistamine drug Hydroxyzine at a glassy carbon electrode: quantification and pharmacokinetic studies. Talanta 74(4):851–859. https://doi.org/10.1016/j.talanta.2007.07.009
British Pharmacopoeia Commission (2020) British Pharmacopoeia. Stationery Office, London. Available from: https://www.pharmacopoeia.com/
European Pharmacopoeia 10th Ed (2020) European directorate for the quality of medicines, Strasbourg. Available from: https://pheur.edqm.eu/home
Kintz P, Godelar B, Mangin P (1990) Gas chromatographic identification and quantification of Hydroxyzine: application in a fatal self-poisoning. Forensic Sci Int 48:139–143. https://doi.org/10.1016/0379-0738(90)90106-9
Odeneal NG II, Casale JF, Wojno HL (2004) Technical Note. Hydroxyzine: an analytical profile. Microgram J. 2:17–21
Rajendraprasad N, Basavaiah K, Vinay K (2011) Optimized and validated spectrophotometric methods for the determination of Hydroxyzine hydrochloride in pharmaceuticals and urine using iodine and picric acid. J Serb Chem Soc 76:1551–1560. https://doi.org/10.2298/jsc101007138r
Rajendraprasad N, Basavaiah K (2013) Titrimetric assay of hydroxyzine dihydrochloride in pharmaceuticals and formulations in non-aqueous medium. Int J Chem Tech Res 5(1):105–111
Rajendraprasad N, Basavaiah K, Vinay B (2010) Acid-base titrimetric assay of Hydroxyzine dihydrochloride in pharmaceutical samples. Chem Ind Chem Eng Q 16(2):127–132. https://doi.org/10.2298/ciceq090929014r
Saeed N, Ali RF (2011) Chiral separation and quantitation of cetirizine and Hydroxyzine by maltodextrin-mediated CE in human plasma: effect of zwitterionic property of cetirizine on enantioseparation. Electrophoresis 32:764–771. https://doi.org/10.1002/elps.201000607
Sawantdesai NS, Kale PP, Savai J (2016) Evaluation of anxiolytic effects of aripiprazole and Hydroxyzine as a combination in mice. J Basic Clin Pharm 7(4):97–104. https://doi.org/10.4103/0976-0105.189429
Sher N, Siddiqui FA, Fatima N, Perveen S, Shafi N (2014) New method development for Hydroxyzine determination: application in stability studies, pharmaceutical formulations, and humane serum. J Liq Chromatogr Relat 38(8):911–918. https://doi.org/10.1080/10826076.2014.991871
United States Pharmacopoeia 43 National Formulary 38 (2019) United States pharmacopeial convention, Rockville. Available from: https://www.usp.org/
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
II and MB conducted the experiments and analyzed the data. VY, VM, OK assisted in the experiments and discussed the results. MB and II wrote the manuscript and drew the graphs. VM, OK revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iurchenko, I., Blazheyevskiy, M., Yaremenko, V. et al. Titrimetric determination of Hydroxyzine using Oxone. Chem. Pap. 77, 5893–5899 (2023). https://doi.org/10.1007/s11696-023-02908-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02908-y