Abstract
The investigated cast Ni50–Ti50 shape memory alloy was prepared using a vacuum arc furnace. The cast samples were subjected to in-vitro biocompatibility studies according to ISO 10993-12:2004, and compared to other samples Ni–Ti orthodontic wires commercially available at the dental market. The cast samples were hydroxyapatite-coated using the electrodeposition technique. The effect of surface treatment on the coating quality was addressed. The hydroxyapatite-coated samples were investigated using electrochemical impedance (EIS) and potentiodynamic techniques. Coated samples were also examined using a scanning electron microscope to inspect the coating morphology. Cytotoxicity tests on MG63 and H9C2 cell lines showed the safety and biocompatibility of the cast NiTi alloy, with a direct relationship between the incubation period of the tested samples and cell viability. Well-adhered hydroxyapatite coating was obtained on the surface-treated NiTi samples using the electrodeposition technique. EDS analysis showed a hydroxyapatite coating having a calcium to phosphorus ratio close to that of the natural bone. Electrochemical tests indicated that the highest corrosion resistance was obtained for the uncoated samples followed by the anodized sample and finally the hydroxyapatite-coated samples due to their high porosity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metallic alloys used in fabricating dental and biomedical appliances must fulfill certain requirements regarding strength, hardness, and resilience. However, biocompatibility is considered the most required property in medical appliances, due to their direct contact and interaction with the living tissues (Eliades, 2007). Nickel-titanium (NiTi or Nitinol) alloy has attracted orthodontists and endodontists for its superelasticity and smart shape memory effect. These effects are employed in multiple dental appliances such as archwires, coil springs, endodontic files, and other auxiliary devices that are used in the dental field (Otsuka et al. 1998). The superior mechanical properties of Nitinol are principally due to its ability to undergo martensite–austenite phase transformation. This transformation is induced by temperature and/or stress stimulus present repeatedly in the oral cavity (Bushow, 2001).
Despite all these attractive properties, some concerns regarding their biocompatibility arose due to the release of nickel element, the main constituent of NiTi alloy, which showed undesirable biological effects, (Shabalovskaya, 2002) in contrast to titanium which showed good biocompatible effects (Abdel-Salam et al. 2019; El-Hadad et al. 2020). The biological concern of the Nickel ions arose from the fact that these ions antagonize essential metal ions, such as magnesium, calcium, and zinc ions, that are involved in several biological processes such as protein synthesis and cell replication (Haider et al. 2009, 2010; Shih et al. 2000b, a).
Due to the strong interatomic bond between nickel and titanium ions in NiTi alloys, as well as, the formed protective TiO2 oxide layer on the alloy’s surface, nickel ions are not easily released into the physiological media (Williams, 1981). This clarifies the reason why many research studies have confirmed the biocompatibility of NiTi (Shabalovskaya, 2002). Nevertheless, several studies reported some biological adverse effects for NiTi alloys. This may be due to the breaking of the oxide film and substantial nickel ions released in the adjacent tissues (Assad et al. 1994; Castelman et al. 1981). However, various experimental researches examined the biocompatibility of polycrystalline form of Nitinol and proved their nontoxicity and suitability for use in several biological fields (Shih et al. 2000b, a; Rose et al. 1998; Trepanier et al. 1999; Ryha¨nen et al. 1999). On the other hand, there is little information about the single crystalline form of Nitinol, though their mechanical properties benefit over the polycrystalline form (Sehitoglu et al. 2001; Gall et al. 2002; Aziza et al. 2003). Still, there is no agreed opinion concerning the cytotoxic effect of the nickel ion released from various NiTi alloys in the biological environment (Alina et al. 2015).
As a solution to prevent ions release, a chemically stable and biocompatible coating on NiTi is thought to be effective. Hydroxyapatite is well-known as the most popular biocompatible coating that reduces or eliminates the release of ions (Koumya et al. 2021; Gkomozaa et al. 2019; Chen et al. 2019; Vilardella et al. 2020). Hydroxyapatite coating is applied on the titanium alloys using various techniques including: hydrothermal method, solid-state reaction, co-precipitation, sol–gel, sputtering, mechanochemical, mechanochemical-hydrothermal, microemulsion, and others. The challenge in this respect is to have an economically feasible method for applying hydroxyapatite and to determine the time to acquire the desired properties regarding adhesion and bioactivity (Swarnima et al. 2020; Yu et al. 2019; Bakhsheshi-Rad et al. 2018).
In the current investigation, in vitro biocompatibility of cast Ni–Ti alloy was studied in terms of cytotoxicity evaluation and ion release investigation at different time intervals. Moreover, Nitinol samples were surface treated in different solutions followed by hydroxyapatite coating, and the coat quality as influenced by the surface treatment was investigated. Based on the obtained results, the suitability of the vacuum arc melting process for preparing biocompatible Nitinol alloys was assessed.
Materials and methods
Preparation of cast NiTi alloy
High purity titanium (Ti) and Nickle (99.9%) were used for preparing the cast ingots. The investigated cast Ni–Ti shape memory alloy was prepared as Ni (50 at.%) and Ti (50 at.%). Before melting, the raw materials were cleaned using ultrasonic to remove dirt. The samples were prepared by melting using a vacuum arc furnace (model LHD 1250) for avoiding titanium reactivity. Photographs of the vacuum melting furnace and melting chamber are attached as supporting files. The furnace has three main units: melting chamber, control unit, and chiller. As the first step, pieces of pure metals (60 gm of Ni and 60 gm of Ti) were placed in a water-cooled crucible and the melting chamber was evacuated. When the vacuum reached the required degree, argon gas was injected into the vacuum chamber then the high voltage was applied between the copper crucible and the arc gun. Once the arc touched the raw material, the arc was initiated. Electromagnetic stirring was applied to ensure homogeneity of the cast samples. Finally, a disc sample of 80 mm diameter with an average thickness 10 mm-mm was obtained.
In-vitro biocompatibility tests
The in-vitro biocompatibility tests were conducted according to the ethical guidelines of the medical research ethics committee of the national research centre, with approval number (9,817,082,022).
Sample preparation and sterilization for testing
The cast Ni–Ti samples, Fig. 1, were prepared according to the stated dimensions in (ISO) 10993-12:2004 specification (International Organization for Standardization (ISO)10993-12:2004—Part 12) via a high precision micro cutter (Pico175) under a copious amount of water, resulting in a rod size of approximately 2 mm in diameter. NiTi orthodontic wires (Ni–Ti Superelastic Natural orthodontic arch wires—Astar Orthodontic Inc. Shanghai China) commercially available in dental market were used as a reference control for the in-vitro biocompatibility test as stated by the International Organization for Standardization (ISO) 10993-12:2004 (International Organization for Standardization (ISO)10993-12:2004—Part 12). The two group samples (Table 1) were ultrasonically cleaned for fifteen minutes in distilled water at room temperature using ultrasonic cleaner (Daihan scientific Ultrasonic cleaner. Korea), to remove any impurities. Hence, the samples were dried and steam-sterilized at 121 °C for 20 min using autoclave (Systec GmbH, Germany).
Cell culture
Cell culture tests were performed according to (ISO) 10993-5:2009 (International Organization for Standardization (ISO) 10993-5:2009—Part 5). Experimentally prepared NiTi specimens (sample 1), as well as, the reference control specimens (sample 2) were soaked for 24 h in sterilized phosphate buffer saline (PBS) that was supplemented with 100 units/ml penicillin G sodium, 250 mg/ml amphotericin B, 100 units/ml streptomycin sulfate and cell expansion medium, before the cytocompatibility study, as a treatment step (Teng et al. 2002).
Two different cell lines; human osteoblast-like cell (MG63), and human cardiac cell (H9C2) purchased from ATCC, USA, were separately cultured in McCoy's 5a medium supplemented with 2 mM L-glutamine, 100 units/mL penicillin G sodium, 250 mg/mL amphotericin B, 100 units/mL streptomycin sulfate, and 10% fetal bovine serum (FBS). Cells were maintained at sub-confluency at 37 °C in humidified air containing 5% CO2. For sub-culturing, monolayer cells were harvested after trypsin/EDTA treatment at 37 °C. Cells were used when confluence had reached 75%. All cell culture materials were obtained from Cambrex BioScience (Copenhagen, Denmark). All chemicals were brought from Sigma Aldrich, USA.
Cytotoxicity evaluation using MTT assay
The metabolic cell viability of sample 1 and sample 2, in the vicinity of the separately cultured MG63 and H9C2 cell lines (1 × 104 cells/well), was determined using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (Hansen et al. 1989). Based on the observation that a mitochondrial enzyme of viable cells can metabolize a water-soluble tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide into an insoluble formazan salt). The number of viable cells is directly proportional to the level of soluble formazan dark blue color. The extent of the reduction of MTT was quantified by measuring the absorbance at 570 nm. Sample 1 and sample 2 were applied in replicates. Control wells were left without adding any tested materials. Results were obtained at 1, 3 and 5-day incubation intervals and statistical analysis of the results was performed using Graph pad prism 9.3 ANOVA test.
Cell growth pattern and mode of cell death in the vicinity of Ni–Ti samples
Apoptosis/Necrosis stain (ethidium bromide/acridine orange) test method (Kasibhatla et al. 2006) was used to screen the effect of the experimental NiTi specimens (sample 1) on the growth pattern and mode of cell death of the MG-63 cell line in comparison to the reference commercial control specimens (sample 2) at different time points. Cells were incubated for 1, 3, and 5 day time intervals with the specimens of samples 1 and sample 2 in cell culture slides (5 × 104 cells/ chamber, SPL Life Sciences, Korea). The control cell culture slides were left without any added samples.
Slides were washed with Phosphate-Buffered Saline (PBS) and stained with acridine orange and ethidium bromide (AO/EB, 100 µg/ml in PBS) for 10 min in dark. Acridine orange is a vital dye that stains both live and dead cells. Ethidium bromide stains only cells that have lost membrane integrity. Vital cells appear uniformly green, while dead cells stain orange due to the incorporation of ethidium bromide and acridine orange in dead cells.
Stained cells were examined under a fluorescence microscope (Axio Imager Z2, Zeiss, Jena, Germany) at a magnification of 20x, with a fluorescent camera (AxioCam MRc3 S/N 4299, Carl Zeiss Microscopy GmbH (Jena, Germany).) and ZEN 11 blue edition software for Image Analysis (Zeiss, Jena, Germany). Percentages of living, early apoptotic, late apoptotic, and necrotic cells were calculated for each concentration and compared to the untreated control cells.
Metal ion release in different time intervals
Nickel and titanium ions released in cell culture media (DMEM supplied by Sigma) from both sample 1 and sample 2 after 1, 3, and 5 days of immersion were analyzed using inductively coupled plasma atomic emission spectroscopy (ICP-AES) using Agilent 5100 Synchronous Vertical Dual View (SVDV). DMEM cell culture medium was used as a negative control.
Influence of surface treatment on quality of hydroxyapatite coating
Surface treatment process
Rectangular samples measuring 4.5 cm x 1 cm x 1 cm were machined for coating experiments. Samples were polished with emery papers up to 2500 and then polished with alumina so as to acquire a mirror-like finish, then washed with distilled water, rinsed in acetone for 10 min. and finally washed with distilled water. Samples were surface treated prior to coating in two steps:
-
Pickling treatment: samples were pickled in 50% phosphoric at room temperature or in boiling using 37% hydrochloric acid.
-
Activation treatment: pickled samples were activated in hydrofluoric acid at different concentrations.
Finally, dried samples were weighted using digital panels.
Coating by hydroxyapatite
A coating bath containing 75 ml CaCl2 (0.167 M), 75 ml NH4H2PO4 (0.1 M), and 10 ml H2O2 (600 ppm) was used for coating the investigating Ni–Ti samples with hydroxyapatite. The electrodeposition was performed using a conventional electrochemical cell equipped with three electrodes; platinum, calomel, and Ni–Ti alloy served as the counter, the reference, and the working electrode, respectively. In the electrodeposition experiments, a current of 30 mA/cm2 was applied to the sample with an area of 19 cm2 so as to impose a current density of 1.5 A/cm2 at room temperature for one hour using a computerized potentiostat. After the electrodeposition, the samples were put in 4% NaOH for at least 20 min at 80 °C to allow the transformation of brushite (phosphate mineral) to hydroxyapatite.
Electrochemical characterization of coated samples
Electrochemical impedance (EIS) and potentiodynamic techniques were used for evaluating the electrochemical behavior of uncoated and coated samples. Tests were performed using three-electrode corrosion cells. The coated sample was used as a working electrode with a 0.2 cm2 exposed area. A saturated calomel electrode (SCE) was used as a reference electrode, and a platinum sheet as a counter electrode. Tests were conducted using a computerized potentiostat (Auto lab PGSTAT 30). EIS experiments were performed at room temperature over a frequency range between 1.0 Hz and 100 kHz at open circuit potential with 0.01 V amplitude voltage signal. Potentiodynamic polarization tests were carried out at potentials from − 0.3 to 1.5 V against open-circuit potential (OCP) values for cathodic and anodic scans, respectively. The test was carried out for 1 h immersion in a test electrolyte with a potential step of 0.001 V and a scan rate of 0.001 V/s using Auto lab PGSTAT 302 N potentiostat. Experiments were performed in simulated body fluid (Hank’s physiological solution) having a chemical composition given in Table 2.
Results and discussion
Microstructure of cast nitinol
The phase diagram of Ni–Ti binary system is presented in Fig. 2a (Otsuka et al. 1999). Optical microstructure of the current cast Ni–Ti specimen showed a dendritic structure, as shown in Fig. 2b. The microstructure of the investigated Ni–Ti cast sample is composed of NiTi matrix that corresponds to the intermetallic phase TiNi and Ti3Ni4 with a few Ti2Ni particles that are normally existing frequently in such cast Ni–Ti structure.
Results of in-vitro cell culture test
Nitinol usage for biomedical and dental appliances has received significant attention due to its smart mechanical and corrosion resistance properties, though their concerns about the potential release of nickel in the human body. In this study, vacuum melting process was conducted for preparing the biocompatible Nitinol alloy with a composition of Ni–Ti (50–50 at%). To evaluate the effect of this technique on the biocompatibility of Nitinol alloy, MTT cytotoxicity test, as well as, metal ions released into the incubation solution at different time intervals were assessed.
MTT cytotoxicity evaluation
MTT cytotoxicity test was carried out using two different cell lines MG63 and H9C2. The results showed that both sample 1 (Experimentally prepared NiTi specimens) and sample 2 (the reference control specimens) had no adverse cytotoxic effects on any of the tested cell lines at all the tested time intervals, verifying their cytocompatibility (Fig. 3). There were direct relationships between cell viability of both samples and incubation period of the tested samples, where, as the incubation period increases, cell viability significantly increases from day 1 to day 5 incubation period, for sample 1 and sample 2, for both MG63 and H9C2 at p < 0.01, p < 0.05, p < 0.001 and p < 0.0001, respectively. It was obvious that there was no significant difference between sample1 and sample 2 when inspecting the viability of MG63 cell line at 1, 3 and 5 days’ incubation period, as well as, when inspecting the viability of H9C2 cell line at a 1 day incubation period. Whereas, sample 1 showed significantly higher viability for H9C2 cell line than that of sample 2, at 3 and 5 days’ incubation period at p < 0.01. Previous literature attributed cell viability to the chemical composition of the titanium alloys studied (Velasco-Ortega et al. 2010). Therefore, the obtained results verify that the chemical composition and the manufacturing technique of the casted NiTi alloy didn’t have any adverse effect on its biocompatibility and cell viability. These results go with previous results that revealed no cytotoxic effect for nickel-titanium alloy (Wever et al. 1997; Assad et al. 2002).
Effect of sample 1 (Experimentally prepared NiTi specimens) and sample 2 (the reference control specimens) on MG-63 and H9C2 cells viability at 1, 3 and 5 day time intervals, as estimated by MTT assay. Data are represented as percent of control (untreated negative control cells) [mean ± St. dev]. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Results of mode of cell death in MG-63 cell line at different time points
As shown in Fig. 4 for fluorescence microscope images, sample 1 (Experimentally prepared NiTi specimens) showed increased viability along with the time points when compared to the control group ( MG63 cells with no added test specimen) as revealed by intact membranes with uniform bright green nuclei. Moreover, Sample 1 showed comparable apoptotic and necrotic changes that resemble the control group, revealing a normal death rate. Sample 2 (the reference control specimens), on the other hand, obtained higher viability among different time points with decreasing levels of apoptotic and necrotic changes in comparison to both the control and sample 1. Nevertheless, a previous study stated that direct exposure to titanium particles could induce apoptosis, through stimulating tumor suppressor proteins p53 and p73 and that the degree of stimulation depends on the composition of the alloy and the its incubation period with the cells (Wang et al. 2003).
Fluorescence microscope images showing the mode of cell death of MG63 cell line where; a, b and c represent; control (MG63 cells with no added test specimen) at 1, 3 and 5 days incubation period, respectively. d, e and f represent cells in the vicinity of sample 1 (Experimentally prepared NiTi specimens) at 1, 3 and 5 days incubation period, respectively. g, h and i represent cells in the vicinity of sample 2 (the reference control specimens) at 1, 3 and 5 days incubation period, respectively. Acridine orange/ethidium bromide stain revealed mode of cell death, Magnification 10X. Scale bar 10 μm
Results of metal ion release at different time intervals
Nickel and titanium ions released in cell culture media (DMEM) at 1, 3 and 5 days immersion periods were analyzed using ICP-AES. It is worth mentioning that no titanium ions were detected in the incubation medium from both samples at all incubation intervals. On the other hand, Ni ion released in mg/L for sample 1 (experimentally prepared NiTi specimens) and sample 2 (the reference control specimens) at 1, 3 and 5-day intervals when analyzed was found to be (0.056, 0.083 and 0.09 mg/L) for sample 1 and (0.0533, 0.073and 0.046 mg/L) for sample 2, respectively, Fig. 5.
The result deduces that maximum Ni ion release was for sample 1 at 5 days’ incubation period followed by sample 1 at 3 days’ incubation period and sample 2 at 3 days’ incubation period. Surprisingly, the higher Ni ion release from sample 1 does not induce any cytotoxic effect on both MG-63 and H9C2 cells as revealed by the MTT cytotoxicity test. In addition, it was reported that the normal daily intake of Ni is 0.2–0.3 mg/day, which is much higher than the amount of nickel ion released in the current study. Nevertheless, this comparison is not dependable clinically because the amount of Ni required to create contact hypersensitivity reactions totally relays on human variability (Dunlap et al. 1989).
Optimization of pickling bath and activation bath based on the coating quality
Table 3 gives the coating quality obtained after using different concentrations of HF, H3PO4 and HCl pickling acids. Excellent coating quality regarding coverage and adherence to the substrate was only obtained upon using 37% HCl for (10 min.) and 5% HF for (5 min), whereas good quality coating was obtained. The effect of pickling time was also addressed, where at 5 min excellent coating was obtained. At a current density of 1.5 mA/cm2, rate of OH- formation is enhanced; therefore, the formation rate of the calcium phosphate layer on the Ni–Ti alloy substrate is raised. As a result, a homogenous film can be made. It is noted that activation in 40% HF or 50% phosphoric acid results in formation of poor coating which can be removed by finger nail scratching. In addition, there no coating was observed on the surfaces for the studied samples that did not apply any activation process on their surfaces.
SEM investigation
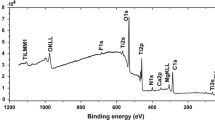
SEM images of the hydroxyapatite coated Ni–Ti samples, before and after soaking in sodium hydroxide, are shown in Fig. 6. Before soaking in sodium hydroxide, flake-like particles are formed and consequently cover the whole sample surface (Fig. 6a,b). After soaking in sodium hydroxide, the number of flakes were decreased and fine precipitates forming instead (Fig. 6c,d). The thickness of the hydroxyapatite coating layer was determined by investigating the cross section of hydroxyapatite coated Ni–Ti sample under the digital optical microscope (Fig. 6e). The measured thickness was about 62 microns. EDS analysis of the hydroxyapatite coating formed on the Ni–Ti alloy sample after soaking in sodium hydroxide (Fig. 7) revealed a Ca to P atomic ratio of 1.77 which is close to the Ca to P atomic ratio of hydroxyapatite forming the natural bone material (1.67). It is well known that the electrodeposition process leads to the formation of CaHPO4 × 2H2O (calcium hydrogen phosphate dihydrate) and Ca3(PO4)2 (calcium phosphate), which further transform into more stable HAP after the NaOH treatment (Chen et al. 2003).
Potentiodynamic polarization test
Potentiodynamic polarization curves of uncoated and hydroxyapatite coated Ni–Ti samples are shown in Fig. 8. The uncoated sample obtained noticeably low current density compared to the coated one which can be related to the porosity of the hydroxyapatite coating. The porous coating allows penetration of testing fluid and hence flowing of current. On the other hand, the uncoated sample did not suffer from corrosion attack due to titanium oxide protective film that formed on the alloy surface. The electrochemical parameters, namely corrosion potential (Ecorr), corrosion current density (icorr) and corrosion rate, were extracted from the curves and given in Table 3. The corrosion rate of the investigated samples was calculated according to equation [1]. The equivalent weight of the alloy was calculated as follows:
For pure elements, the equivalent weight is given by:
where: W is the atomic weight of the element, and n is the number of electrons required to oxidize an atom of the element in the corrosion process, which is, the valence of the element.
On the other hand, the calculation of the equivalent weight of alloys is more complex, because the process of oxidation occurs selectively on some component of the alloy, thus the calculation approach must be adjusted to reflect the observed mechanism. Furthermore, many elements display more than one valence value, therefore some rationale must be implemented for assigning values of n to the existing elements in the alloy.
To calculate the alloy equivalent weight, the following approach may be used (Aniołek et al. 2021). Consider a unit mass of alloy oxidized. The electron equivalent for 1 g of an alloy, Q, is given by Eq. 2:
where: fi = mass fraction of the ith element in the alloy, Wi = atomic weight of the ith element in the alloy, and ni = the valence of the ith element of the alloy.
Therefore, the alloy equivalent weight, EW, is the reciprocal of this quantity:
The corrosion parameters of uncoated and hydroxyapatite coated samples are given in Table 4. The corrosion rate of the uncoated sample (0.0014 mm/y) is much lower than the corrosion rate of the hydroxyapatite coated sample (0.1178 mm/y). This is expected because of the fact that the hydroxyapatite layer is porous allowing the penetration of the testing fluid causing the deterioration of the hydroxyapatite layer.
EIS results
Figure 9 illustrates bode curves of uncoated and coated samples. The curves were analyzed using the fit and simulate option in the potentiostat software (Table 4). The highest corrosion resistance was obtained for the uncoated Ni–Ti where an impedance was registered as high as 70 KΩ, while a markedly lower value of 30 KΩ was obtained for the hydroxyapatite coated sample. In the same manner, a higher phase angle (≈66°) was shown for the uncoated sample compared to about 50° that was observed for the coated sample. These results for the investigated Ni–Ti samples substantiate the results of the potentiodynamic polarization test. A simple equivalent circuit can be used to simulate the EIS behavior as seen in Fig. 10, where Rs is the solution resistance, CPE represents the constant phase element corresponding to the capacitance of the electrode surface, and Rp is the polarization resistance (Abdulhamid et al. 2018).
Conclusions
In the current investigation, Ni–Ti alloy was prepared using vacuum arc melting furnace and its biocompatibility was evaluated in terms of cytotoxicity, ion release and hydroxyapatite precipitation. The following points can be concluded as follows:
-
Cytotoxicity tests on MG63 and H9C2 cell lines showed safety and biocompatibility of the studied Ni–Ti alloy in the as-cast condition.
-
The amount of Nickel ion release from the investigated Ni–Ti alloy was well matched within the safety limit.
-
Well adhered hydroxyapatite coating was achieved for Ni–Ti sample by applying electrodeposition using a coating bath containing calcium and phosphate ions under 10 mA/cm2 at room temperature for one hour. The best coating process was achieved for the sample that was activated by [HCl + 5% HF].
-
Electrochemical tests indicated that the highest corrosion resistance was obtained for the uncoated Ni–Ti sample followed by the anodized sample and finally the hydroxyapatite coated sample due to its high porosity.
References
Abdel-Salam M, El-Hadad S, Khalifa W (2019) Effects of microstructure and alloy composition on hydroxyapatite precipitation on alkaline treated α/β titanium alloys. Materials Science and Engineering C 104: no. 109974.
Abdulhamid SA, Ghayad IM, Kandemirli F (2018) Electroless plating of Ni-B composite coating on AZ31 Mg alloy. Inter Cast Metal Res 10:11–19
Alina S, Alexandru S, Tudor P, Alina M, Zetui IN (2015) Assessment of orthodontic biomaterials’ cytotoxicity: an in vitro study on cell culture. Rom J Morphol Embryol 56:1119–1125
Aniołek K, Łosiewicz B, Kubisztal J, Osak P, Stróz A, Barylski A, Kaptacz S (2021) Mechanical properties, corrosion resistance and bioactivity of oxide layers formed by isothermal oxidation of Ti-6Al-7Nb alloy. Coatings 11:505
Assad M, LombardiBerne`che S, Desrosiers EA, Yahia L’H, Rivard CH, S (1994) Essais de cytotoxicite´ sur l’alliage a` me´moire de forme nickel–titane. Ann Chir 48:731–736
Assad M, Chernyshov A, Leroux MA, Rivard CH (2002) A new porous titanium–nickel alloy: part 1. Cytotoxicity and genotoxicity evaluation. Biomed Mater Eng 12:225–237
Aziza M, Fatiha C, Yahye M, Yuriy C, Hocine Y (2003) In vitro cytotoxicity evaluation of a 50.8% NiTi single crystal. Wiley Periodicals, Inc.
Bakhsheshi-Rad HR, Hamzah E, Ismail AF, Aziz M, Karamian E, Iqbal N (2018) Bioactivity, in-vitro corrosion behavior, and antibacterial activity of silver–zeolites doped hydroxyapatite coating on magnesium alloy. Trans Nonferrous Met Soc China 28:1553–1562
Bushow Kh (2001) The encyclopedia of materials science and technology. Elsevier Science, New York
Castelman LS, Motzkin SM (1981) The biocompatibility of nitinol. In: Williams DF, editor. Biocompatibility of clinical implant materials. Boca Raton, Florida: CRC Press 1: 129–154.
Chen MF, Yang XJ, Liu Y, Zhu SL, Cui ZD, Man HC (2003) Study on the formation of an apatite layer on NiTi shape memory alloy using a chemical treatment method. Surf Coat Technol 173:229–234
Chen QY, Zou YL, Fu W, Bai XB, Ji GC, Yao HL, Wang HT, Wang F (2019) Wear behavior of plasma sprayed hydroxyapatite bioceramic coating in simulated body fluid. Ceram Int 45:4526–4534
Dunlap CL, Vincent SK, Barker BF (1989) Allergic reaction to orthodontic wire: report of case. J Am Dent Assoc 118:449–450
El-Hadad S, Ghaith M, Yassin A, Kamal M (2020) Optimizing the surface treatment processes to enhance the bioactivity of Ti–6Al–7Nb alloy. Trans Indian Inst Met 73:2727–2738
Eliades T (2007) Orthodontic materials research and applications: part 2. Current status and projected future developments in materials and biocompatibility. Am J Orthod Dentofacial Orthop 131:253–262
Gall K, Dunn M, Liu Y, Labossiere P, Sehitoglu H, Chumlyakov Y (2002) Micro and macro deformation of single crystal NiTi. Trans Am Soc Mech Eng 124:238–245
Gkomozaa P, Vardavouliasa M, Pantelisb DI, Sarafogloub CH (2019) Comparative study of structure and properties of thermal spray coatings using conventional and nanostructured hydroxyapatite powder, for applications in medical implants. Surf Coat Technol 357:748–758
Haider W, Munroe N, Pulletikurthi C, Gill P, Amruthaluri S (2009) A comparative biocompatibility of ternary nitinol alloys. J Mater Eng Perform 18:765–767
Haider W, Munroe N, Tek V, Pulletikurthi C, Puneet K, Gill S, Pandya S (2010) Review on surface modifications of nitinol. J Long Term Effects Med Implant 19:15–24
Hansen MB, Nielsen SE, Berg K (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Method 119:203–210
International Organization for Standardization (ISO) 10993–5:2009 Biological evaluation of medical devices—part 5: tests for in vitro cytotoxicity.
International Organization for Standardization (ISO)10993–12:2004. Biological evaluation of medical devices—part 12: Sample preparation and reference materials.
Kasibhatla S, Amarante-Mendes GB, Finucane D, Brunner T, Bossy-Wetzel E, Green DR (2006) Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis Cold Spring Harbor Protocols: 21.
Koumya Y, Ait Salam Y, Khadiri ME (2021) Pitting corrosion behavior of SS-316L in simulated body fluid and electrochemically assisted deposition of hydroxyapatite coating. Chem Pap 75:2667–2682
Otsuka K, Ren X (1999) Martensitic transformations in nonferrous shape memory alloys. Mater Sci Eng, A 273–275:89–105
Otsuka K, Wayman CM (1998) Shape memory materials. Cambridge University Press, New York, pp 27–47
Rose EC, Jonas IE, Kappert HF (1998) In vitro investigation into the biological assessment of orthodontic wires. J Orofac Orthop 59:253–264
Ryha¨nen J, Kallioinen M, Serlo W, Pera¨ma¨ki P, Junior J, Sandvik P, Niemela¨ E, Tuukkanen J (1999) Bone healing and mineralization, implant corrosion, and trace metals after nickel-titanium shape memory metal intramedullary fixation. J Biomed Mater Res 47: 472– 480.
Sehitoglu H, Jun J, Zhang X, Karaman I, Chumlyakov Y, Maier HJ, Gall K (2001) Shape memory and pseudoelastic behavior of 51.5% Ni-Ti single crystals in the solutionized and overaged state. Acta Mater 49:3609–3620
Shabalovskaya SA (2002) Surface, corrosion and biocompatibility aspects of nitinol as an implant material. Bio-Med Mater Eng 12:69–109
Shih C-C, Lin S-J, Chen Y-L, Su Y-Y, Lai S-T, Wu GJ, Kwok C-F, Chung K-H (2000a) The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J Biomed Mater Res A 52:395–403
Shih CC, Lin SJ, Chen YL, Su YY, Lai ST, Wu GJ, Kwok CF, Chung Kh (2000b) The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J Biomed Mater Res 52:395–403
Swarnima S, Krishna KP, Aminul I, Anup KK (2020) Corrosion behavior of plasma sprayed graphene nanoplatelets reinforced hydroxyapatite composite coatings in simulated body fluid. Ceram Int 46:13539–13548
Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D (2002) Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. PNAS 99:3024–3029
Trepanier C, Leung TK, Tabrizian M, Yahia L’H, Bienvenu JG, Tanguay JF, Piron DL, Bilodeau L, (1999) Preliminary investigation of the effects of surface treatments on the biological response to shape memory NiTi stents. J Biomed Mater Res 48:165–171
Velasco-Ortega E, Jos A, Cameán AM, Pato-Mourelo J, Segura-Egea JJ (2010) In vitro evaluation of cytotoxicity and genotoxicity of a commercial titanium alloy for dental implantology. Mutat Res 702:17–23
Vilardella AM, Cincaa N, Garcia-Giraltb N, Dostaa S, Canoa IG, Noguésb X, Guilemany JM (2020) In-vitro comparison of hydroxyapatite coatings obtained by cold spray and conventional thermal spray technologies. Mater Sci Eng, C 107:110306
Wang ML, Tuli R, Manner PA, Sharkey PF, Hall DJ, Tuan RS (2003) Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J Orthop Res 21:697–707
Wever DJ, Veldhuizen AG, Sanders MM, Schakenraad JM, Van Horn JR (1997) Cytotoxic, allergic and genotoxic activity of a nickel–titanium alloy. Biomaterials 18:1115–1120
Williams DF, Biocompatibility of clinical implant materials, (1981) Boca Raton. CRC Press, Vol, Florida, p 1
Yu B, Bai-xiang C, Wen M, Cai-wen L (2019) Suspension plasma-sprayed fluoridated hydroxyapatite coatings: effects of spraying power on microstructure, chemical stability and antibacterial activity. Surf Coat Technol 361:222–230
Acknowledgements
The authors would like to acknowledge the financial support from the Science and Technology Development Fund, Grant No. 37079.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, K.M., Safwat, E.M., Ghayad, I.M. et al. In-vitro biocompatibility evaluation of cast Ni–Ti alloy produced by vacuum arc melting technique for biomedical and dental applications. Chem. Pap. 77, 847–858 (2023). https://doi.org/10.1007/s11696-022-02523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02523-3