Abstract
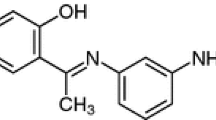
The influences of the application of mono- and two-component organic modifiers on lipophilicity determination of 12 tetradentate Schiff bases by reversed-phase thin layer chromatography were investigated. The main goal is to estimate types of interaction between observed compounds and components of the applied chromatographic systems and establish some behaviour pattern in order to easier choose a combination of organic modifiers which will simulate interaction in biological systems based on the facts that the same basic intermolecular interactions are responsible for the behaviour of substances in both the biological and chromatographic system. The applied organic modifier shows the ability to modify the surface of the applied sorbent, which affects the manifestation of lipophilicity of the observed compounds. Mono-component organic modifiers from different groups of the Snyder triangle were used, as well as their two-component mixtures. In addition, we compared experimentally determined calculated parameters of lipophilicity.
Similar content being viewed by others
References
Aburas N, Lolić A, Stevanović N, Tripković T, Nikolić-Mandić S, Baošić R (2012) electrochemical behavior and antioxidant activity of tetradentate Schiff bases and their copper(II) complexes. J Iran Chem Soc 9(6):859–864
Aburas NM, Stevanović NR, Milčić MK, Lolić AD, Natić MM, Tešić ŽL, Baošić RM (2013) Influence of the structure on the antioxidant activity of tetradentate Schiff bases and their copper(II) complexes: possible mechanisms. J Braz Chem Soc 24(8):1322–1328
Anderson OP, La Cour A, Findeisen M, Hennig L, Simonsen O, Taylor LF, Toftlund H (1997) Zinc(II) N2S2 Schiff-Base complexes incorporating pyrazole: syntheses, characterization, tautomeric equilibria and racemization kinetics. J Chem Soc Dalton Trans 1:111–120
Apostolov S, Vastag G, Matijević B, Daković-Sekulić T, Marinković A (2020) Thin-layer chromatography on reversed phase in the characterization of retention behaviour, lipophilicity, and pharmacokinetics of cyanoacetamide derivatives. J Chil Chem Soc 65(1):4654–4660
Baošić R, Milojković-Opsenica D, Tešić Z (2003) The effect of the substituents of β-ketoiminato ligand of copper(II) and nickel(II) complexes on their retention on thin layers of polyacrylonitrile. J Planar Chromatogr Modern TLC 16(6):412–416
Baošić R, Radojević A, Radulović M, Miletić S, Natić M, Tešić Z (2008) Relationships between structure, retention and biological activity of some schiff base ligands and their complexes. Biomed Chromatogr 22(4):379–386
Bate-Smith EC, Westall RG (1950) Chromatographic behaviour and chemical structure I. Some naturally occuring phenolic substances. BBA Biochimica et Biophysica Acta 4(C):427–440
Blagus A, Cinčić D, Friščić T, Kaitner B, Stilinović V (2010) Schiff bases derived from hydroxyaryl aldehydes: molecular and crystal structure, tautomerism, quinoid effect, coordination compounds. Maced J Chem Chem Eng 29(2):117–138
Brzezińska E, Kośka G (2006) A structure-activity relationship study of compounds with antihistamine activity. Biomed Chromatogr 20(10):1004–1016
Cserháti T (1984) Determination of the lipophilicity of some aniline derivatives by reversed-phase thin-layer chromatography. the effect of the organic phase in the eluent. Chromatographia 18(6):318–322
Dugas H, Penney C (1981) Bioorganic chemistry. Springer, New York
Hansch C, Fujita T (1964) ρ-σ-π analysis. a method for the correlation of biological activity and chemical structure. J Am Chem Soc 86(8):1616–1626
Hawrył, AM, Popiołek ŁP, Hawrył MA, Wieboda RS, Niejedli MA (2015) Chromatographic and calculation methods for analysis of the lipophilicity of newly synthesized thiosemicarbazides and their cyclic analogues 1,2,4-triazol-3-thiones. J Braz Chem Soc 26(8):1617–1624
Henchoz Y, Guillarme D, Rudaz S, Veuthey J-L, Carrupt P-A (2008) High-throughput log p determination by ultraperformance liquid chromatography: a convenient tool for medicinal chemists. J Med Chem 51(3):396–399
Henchoz Y, Bard B, Guillarme D, Carrupt PA, Veuthey JL, Martel S (2009) Analytical tools for the physicochemical profiling of drug candidates to predict absorption/distribution. Anal Bioanal Chem 394(3):707–729
Hill AP, Young RJ (2010) Getting physical in drug discovery: a contemporary perspective on solubility and hydrophobicity. Drug Discovery Today 15(15–16):648–655
Hintze, J. 2001. “NCSS and PASS Number Cruncher Statistical Systems.” Kaysville.
Hiroshi T (1986) Determination of log poct by high-performance liquid chromatography, and its application in the study of quantitative structure-activity relationships. Quant Struct Act Relat 5:81–88
Jones RD, Summerville DA, Basolo F (1979) Synthetic oxygen carriers related to biological systems. Chem Rev 79(2):139–179
Krasikov VD (2003) Contemporary planar chromatography. J Anal Chem 58(8):706–720
Lide DR (2004) CRC handbook of chemistry and physics, 85th edn. CRC Press, Boca Raton, FL
McCarthy PJ, Hovey RJ, Ueno K, Martell AE (1955) Inner complex chelates. I. Analogs of bis(acetylacetone)ethylenediimine and its metal chelates. J Am Chem Soc 77:5820–5824
Mohamed GG (2006) Synthesis, characterization and biological activity of bis(phenylimine) Schiff base ligands and their metal complexes. Spectrochim Acta Part A Mol Biomol Spectrosc 64(1):188–195
Olive GH, Olive S (1984) The chemistry of the catalyzes hydrogenation of carbon monoxide. Springer, Berlin
Paneth A, Hawrył A, Plech T, Hawrył M, Świeboda R, Janowska D, Wujec M, Paneth P (2017) Lipophilicity studies on thiosemicarbazide derivatives. Molecules 22(6):952
Perušković DS, Darić B, Blagus A, Stevanović NR, Pavlović AV, Lolić AD, Baošić RM (2015) Influence of organic modifiers on RP-TLC determination of lipophilicity of some polydentate Schiff bases. Monatshefte Fur Chemie 146(1):1–6
Perušković DS, Stevanović NR, Kovačević GN, Stanković DM, Lolić AĐ, Baošić RM (2020) Application of N, N’-bis(acetylacetonato)propylenediimine copper(II) complex as mediator for glucose biosensor. ChemistrySelect 5(5):1671–1675
Raman N, Kulandaisamy A, Thangaraja C, Jeyasubramanian K (2003) Redox and antimicrobial studies of transition metal(II) tetradentate schiff base complexes. Transition Met Chem 28(1):29–36
Schrödinger Release 2017-1 (2017) Ligand docking protocol. Schrödinger, LLC, New York, NY
Rudakov OB, Sedishev IP (2003) Generalized criterion of solvent polarity as a tool for control of chromatographic process. Russian Chem Bull Int Ed 52(1):55–62
Sangster J (1997) Octanol-water partition coefficients: fundamentals and physical chemistry. John Wiley & Sons Inc, Hoboken, NJ, USA
Singh HL, Varshney AK (2006) Synthetic, structural, and biochemical studies of organotin(IV) with Schiff bases having nitrogen and sulphur donor ligands. Bioinorg Chem Appl 2006:1–7
Sławik T, Paw B (2003) RPTLC Determination of the lipophilicity of 1,2-Benzisothiazol-3(2H)-one derivatives substituted in the heterocyclic ring. J Planar Chromatogr - Mod TLC 16(6):442–446
Snyder LR (1974) Classification of the solvent properties of common liquids. J Chromatogr A 92:223–230
Starek M, Komsta Ł, Krzek J (2013) Reversed-phase thin-layer chromatography technique for the comparison of the lipophilicity of selected non-steroidal anti-inflammatory drugs. J Pharm Biomed Anal 85:132–137
Starek M, Plenis A, Zagrobelna M, Dabrowska M (2021) Assessment of lipophilicity descriptors of selected NSAIDs obtained at different TLC stationary phases. Pharmaceutics 13:440–459
Stevanović NR, Perušković DS, Gašić UM, Antunović VR, Lolić VD, Baošić RM (2017) Effect of substituents on prediction of TLC retention of tetra-dentate Schiff bases and their copper(II) and nickel(II) complexes. Biomed Chromatogr 31(3):e3810
Waring MJ (2009) Defining optimum lipophilicity and molecular weight ranges for drug candidates-molecular weight dependent lower Log D Limits based on permeability. Bioorg Med Chem Lett 19(10):2844–2851
Zapała W, Waksmundzka-Hajnos M (2005) Retention process in reversed phase TLC systems with polar bonded stationary phases. J Sep Sci 28(6):566–574
Acknowledgements
This study was supported by the Ministry of Education, Science and Technological Development of Republic of Serbia, Contract number: 451-03-9/2021-14/200168.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stevanović, N., Mijatović, A., Lolić, A. et al. Influence of mono- and two-component organic modifiers on determination of lipophilicity of tetradentate Schiff bases. Chem. Pap. 76, 585–593 (2022). https://doi.org/10.1007/s11696-021-01884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01884-5