Abstract
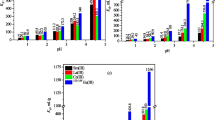
Bentonite dolomite mixture composites were prepared by precipitation technique. The composition of these composite materials was established by different analytical tools; IR, XRD, TGA&DTA, XRF, and BET. X-ray peak broadening analysis was used to evaluate the crystallite size and lattice strain by Scherrer’s formula and Williamson–Hall (W‚ H) analysis. The variation of adsorption of 152+154Eu3+, La3+, Ce3+, and Sm3+ onto studied materials was carried out and the equilibrium time was carried out after 24 h. The capacity of radionuclide (152+154Eu3+) and some lanthanides (La3+, Ce3+, and Sm3+) were investigated, and all studied materials were selective for 152+154Eu3+.




Similar content being viewed by others
References
Abass MR, El-Masry EH, Ibrahim AB (2021) Preparation, characterization, and applications of polyacrylonitrile/ball clay nanocomposite synthesized by gamma radiation. Environ Geochem Health. https://doi.org/10.1007/s10653-021-00813-
Abdel-Galil EA, Ibrahim AB, Abou-Mesalam MM (2016) Sorption behavior of some lanthanides on polyacrylamide stannic molybdophosphate as organic–inorganic composite. Int J Ind Chem 7(3):231–240
Abdou MI, Al-Sabagh AM, Dardir MM (2013) Evaluation of Egyptian bentonite and nano-bentonite as drilling mud. Egypt J Pet 22(1):53–59
Abou-Mesalam M, El-Naggar I (2009) Chemical deposition of zirconium doped tin silicate ion-exchanger and its characterization. J Radioanal Nucl Chem 279(1):333–340
Abou-Mesalam MM (2011) Structural and crystallographic features of chemically synthesized cero-and titanium cero-antimonates inorganic ion exchangers and its applications. Adv Chem Eng Sci 1(01):1–8. https://doi.org/10.4236/aces.2011.11001
Abou-Mesalam MM, Abass MR, Abdel-Wahab MA, Zakaria ES, Hassan AM (2018) Polymeric composite materials based on silicate: II. Sorption and distribution studies of some hazardous metals on irradiated doped polyacrylamide acrylic acid. Desalin Water Treat 109:176–187. https://doi.org/10.5004/dwt.2018.22084
Abou-Mesalam MM, Abass MR, Abdel-Wahab MA, Zakaria ES, Hassan AM, Khalil HF (2016) Complex doping of d-block elements cobalt, nickel and cadmium in magneso-silicate composite and its use in the treatment of aqueous waste. Desalin Water Treat 57(53):25757–25764. https://doi.org/10.1080/19443994.2016.1156031
Abou-Mesalam MM, Abass MR, Ibrahim AB, Zakaria ES (2020) Polymeric composite materials based on silicate. III-Capacity and sorption behavior of some hazardous metals on irradiated doped polyacrylamide acrylonitrile. Desalin Water Treat 193:402–413. https://doi.org/10.5004/dwt.2020.25816
Alabarse FG, Conceição RV, Balzaretti NM, Schenato F, Xavier AM (2011) In-situ FTIR analyses of bentonite under high-pressure. Appl Clay Sci 51(1–2):202–208
Allan KF (2013) Preparation of composite material of polymeric resin mixed with inorganic ion exchanger and their use for the treatment of petrochemical pollutant. Arab J Nucl Sci Appl 46(3):1–13
Andrunik M, Bajda T (2019) Modification of bentonite with cationic and nonionic surfactants: structural and textural features. Materials 12(22):3772
Cao C-Y, Meng L-K, Zhao Y-H (2014) Adsorption of phenol from wastewater by organo-bentonite. Desalin Water Treat 52(19–21):3504–3509
Dongfeng W, Haiyan L, Liyuan W, Zhang L, Ying X (2010) Effects of chitosan-RE3+-bentonite on growth of Chlorella vulgaris. J Rare Earths 28:149–153
El-Aryan YF, Abdel-Galil EA, El-deen GES (2015) Synthesis, characterization and adsorption behavior of cesium, cobalt, and europium on organic-inorganic hybrid exchanger. Russ J Appl Chem 88(3):516–523
El-Didamony H, El-Fadaly E, Amer AA, Abazeed IH (2020) Synthesis and characterization of low cost nanosilica from sodium silicate solution and their applications in ceramic engobes. Boletín de la Sociedad Española de Cerámica y Vidrio 59(1):31–43
El-Naggar IM, Abou-Mesalam MM (2007) Novel inorganic ion exchange materials based on silicates; synthesis, structure and analytical applications of magneso-silicate and magnesium alumino-silicate sorbents. J Hazard Mater 149(3):686–692
Elias A, Abdellah ZN, Villemin D, Didi MA (2017) Effect of microwave irradiations on the sorption of alkylimidazolium salts on bentonite. Chem Pap 71(1):59–65
Espiritu ERL, da Silva GR, Azizi D, Larachi F, Waters KE (2019) Flotation behavior and electronic simulations of rare earth minerals in the presence of dolomite supernatant using sodium oleate collector. J Rare Earths 37(1):101–112
Gao X, Zhong H, Yao G, Guo W, Jin F (2016) Hydrothermal conversion of glucose into organic acids with bentonite as a solid-base catalyst. Catal Today 274:49–54
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190(1–3):916–921
Hamoud MA, Allan KF, Sanad WA, El-Hamouly SH, Ayoub RR (2014) Gamma irradiation induced preparation of poly (acrylamide–itaconic acid)/zirconium hydrous oxide for removal of Cs-134 radionuclide and methylene blue. J Radioanal Nucl Chem 302(1):169–178
Ji J, Ge Y, Balsam W, Damuth JE, Chen J (2009) Rapid identification of dolomite using a Fourier transform infrared spectrophotometer (FTIR): a fast method for identifying Heinrich events in IODP site U1308. Mar Geol 258(1–4):60–68
Kumararaja P, Manjaiah KM, Datta SC, Sarkar B (2017) Remediation of metal contaminated soil by aluminium pillared bentonite: synthesis, characterisation, equilibrium study and plant growth experiment. Appl Clay Sci 137:115–122
Le ZT, Shi L (2012) The effection of copper chloride on the surface of bentonite in adsorption of propylmercaptan. Energy Sources Part A Recov Util Environ Effects 34(13):1231–1237
Meng D, Zhao Q, Pan X (2019) Preparation of La2O3 by ion-exchange membrane electrolysis of LaCl3 aqueous solution. J Rare Earths 37(9):1009–1014
Mohammadi M, Ghaemi A, Torab-Mostaedi M, Asadollahzadeh M, Hemmati A (2015) Adsorption of cadmium(II) and nickel(II) on dolomite powder. Desalin Water Treat 53(1):149–157
Ning Y, Niu S, Zhao S, Zhang X, Zhang Y, Han K et al (2019) Characterization of dolomite promoted by NaAlO2 and application as catalyst in transesterification by response surface methodology. Chem Select 4(33):9849–9856
Nyquist RA, Kagel RO (2012) Handbook of infrared and raman spectra of inorganic compounds and organic salts: infrared spectra of inorganic compounds, vol 4. Academic Press, New York
Palágyi Š (2019) Correction to: Comparison of several models for fitting breakthrough curves of radionuclides transport in crushed rock: groundwater systems. J Radioanal Nucl Chem 322(2):1207–1208
Santos RCR, Vieira RB, Valentini A (2014) Optimization study in biodiesel production via response surface methodology using dolomite as a heterogeneous catalyst. J Catal 2014:1–11
Shady SA (2009) Selectivity of cesium from fission radionuclides using resorcinol–formaldehyde and zirconyl-molybdopyrophosphate as ion-exchangers. J Hazard Mater 167(1–3):947–952
Taha KK, Suleiman TM, Musa MA (2011) Performance of Sudanese activated bentonite in bleaching cottonseed oil. J Bangladesh Chem Soc 24(2):191–201
Tripathy SK, Angadi SI, Patra NK, Rao DS (2018) Comparative separation analysis of direct and reverse flotation of dolomite fines. Miner Process Extr Metall Rev 39(5):339–350
Zhang Y, Ying X, Hao C, Bingjie LIU, Xiang GAO, Dongfeng W, Liang P (2014) La(III)-loaded bentonite/chitosan beads for defluoridation from aqueous solution. J Rare Earths 32(5):458–466
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abass, M.R., Ibrahim, A.B. & Abou-Mesalam, M.M. Retention and selectivity behavior of some lantshanides using bentonite dolomite as a natural material. Chem. Pap. 75, 3751–3759 (2021). https://doi.org/10.1007/s11696-021-01621-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01621-y