Abstract
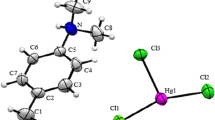
Preparation, structural elucidation, Hirshfeld surface analysis, thermal analysis and impedance spectroscopy study are carried out for an interesting organic–inorganic hybrid compound (NH2C5H3ClNH)2ZnBr4·H2O. X-ray diffraction analysis reveals that the title compound belongs to the triclinic crystallographic system with the space group P-1 with Z = 2 and the following unit cell dimensions: a = 7.3209(9) Å, b = 8.4407(10) Å, c = 16.923(2) Å, α(°) = 82.975(6), β(°) = 85.118(6) and γ(°) = 74.398(6). The crystal structure is composed of two protonated 4-amino-2-chloropyridinium cations, tetrabromozincate anions and a water molecule which are held together by a number of hydrogen bonds forming infinite chains. In addition, crystal structure is stabilized with π…π interactions (Cg–Cg and Zn-Cl…Cg, with Cg is the 4-amino-2-chloropyridinium rings) which have also been investigated in terms of their corresponding Hirshfeld surface and the breakdown of fingerprint. To quantify intermolecular interactions in crystal lattice, FT-IR spectroscopy has been used to distinguish the different chemical functional groups and their environments in this molecule. The dielectric conductivity of this compound has been measured in the temperature range 298–438 K and the frequency range 209 Hz–5 MHz. The analysis of the experimental data of the impedance spectroscopy based on the activation energy shows that this material is an ionic–protonic conductor at low temperature and becomes an electronic one at high temperature. This work aims to reveal the thermal properties of a new zinc (II) based organic–inorganic hybrid and the conductivity properties that this compound exhibits.



















Similar content being viewed by others
References
Böhmer R, Ngai KL, Angell CA, Plazek DJ (1993) Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 99:4201–4209. https://doi.org/10.1063/1.466117
Bidault O, Goux P, Kchikech M, Belkaoumi M, Maglione M (1994) Space charge relaxation in perovskites. J Phys Rev B 49:7868–7873. https://doi.org/10.1103/physrevb.49.7868
Brandenburg K (1998) Diamond version 2.0 impact GbR. Bonn, Germany
Chihaoui N, Hamdi B, Salah AB, Zouari R (2016a) A new mononuclear complex: structure, vibrational (FT-IR and Raman), Hirshfeld surfaces analysis, electrical properties and equivalent circuit. J Phys Chem Biophys 6:216–226. https://doi.org/10.4172/2161-0398.1000216
Chihaoui N, Hamdi B, Dammak T, Zouari R (2016b) Molecular structure, experimental and theoretical spectroscopic characterization and non-linear optical properties studies of a new non-centrosymmetric hybrid material. J Mol Struct 1123:144–152. https://doi.org/10.1016/j.molstruc.2016.06.031
Chihaoui N, Hamdi B, Zouari R (2016c) Structural study, intermolecular interactions in view of X-ray and Hirshfeld surface analysis, DSC characterization, and electrical properties of bis-(4-benzylpyridinium) tetrabromozincate. J Ionics 23:1173–1186. https://doi.org/10.1007/s11581-016-1926-4
Chihaoui N, Hamdi B, Zouari R (2017) Structural study, spectroscopic analysis and dielectric proprieties of new hybrid organic-inorganic compound. J Mol Struct 1147:48–55. https://doi.org/10.1016/j.molstruc.2017.06.087->
Dyre JC, Schrøder TB (2000) Universality of ac conduction in disordered solids. J Rev Modern Phy 72:873–892. https://doi.org/10.1103/revmodphys.72.873
Farrugia LJ (1997) ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Crystallogr 30:565. https://doi.org/10.1107/S0021889897003117
Farrugia LJ (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr 30:837–838. https://doi.org/10.1107/s0021889899006020
Fanggao C, Saunders GA, Lambson EF, Hampton RN, Carini G, Di Marco G, Lanza M (1996) Temperature and frequency dependencies of the complex dielectric constant of poly(ethylene oxide) under hydrostatic pressure. J Polym Sci: Part B: Polym Phys 34:425–433. https://doi.org/10.1002/(SICI)1099-0488(199602)34:3%3c425::AID-POLB3%3e3.0.CO;2-S
Gao FX, Gu W, Yang YS, Qian J, Yan SP (2007) Bis(tetra-n-butylammonium) tetrabromidozincate (II). J Acta Cryst E 63:m1621. https://doi.org/10.1107/s160053680702140x
Gillon AL, Lewis GR, Orpen AG, Rotter S, Starbuck J, Wang X-M, Rodriguez-Martin Y, Ruiz-Pérez C (2000) Organic–inorganic hybrid solids: control of perhalometallate solid state structures. J Chem Soc Dalton Trans. https://doi.org/10.1039/b005036i
Hodge IM, Ngai KL, Moynihan CT (2005) Comments on the electric modulus function. J Non-Cryst Solids 351:104–115. https://doi.org/10.1016/j.jnoncrysol.2004.07.089
James AR, Srinivas K (1999) Low temperature fabrication and impedance spectroscopy of PMN-PT ceramics. J Mater Res Bull 34:1301–1310. https://doi.org/10.1016/s0025-5408(99)00127-0
Jonscher AK (1999) Dielectric relaxation in solids. J Phys D Appl Phys 32:R57–R70. https://doi.org/10.1088/0022-3727/32/14/201
Karaa N, Hamdi B, Ben Salah A, Zouari R (2012) Synthesis, infra-red, MAS-NMR characterization, structural study and electrical properties of the new compound [C5H6ClN2]2Cd3Cl8. J Mol Struct 1013:168–176. https://doi.org/10.1016/j.molstruc.2011.12.053
Karâa N, Hamdi B, Oueslati A, Ben Salah A, Zouari R (2010) Preparation, infra-red, MAS-NMR and structural characterization of a new copper based inorganic–organic hybrid compound: [C5H6N2Cl]2CuCl4. J Inorg Organomet Polym 20:746–754. https://doi.org/10.1007/s10904-010-9409-y
Karâa N, Hamdi B, Ben Salah A, Zouari R (2013) Synthesis, infra-red, CP/MAS-NMR characterization, structural study and electrical properties of the bis(4-amino-2-chloropyridinium) tetrachlorozincate (II) monohydrate. J Mol Struct 1049:48–58. https://doi.org/10.1016/j.molstruc.2013.06.003
Koenderink JJ, van Doorn AJ (1992) Surface shape and curvature scales. Image Vis Comput 10:557–564. https://doi.org/10.1016/0262-8856(92)90076-f
Mesbeh R, Hamdi B, Zouari R (2016) (H2pdcCuBr 2)2∙2(MH +)2Br − (M = Melamine, H2pdc = Pyridine-2,6-Dicarboxylic Acid): crystal structure, Hirshfeld surface analysis, vibrational and thermal studies. J Inorg Organomet Polym 26:744–755. https://doi.org/10.1007/s10904-016-0371-1
Nefzi H, Sediri F, Hamzaoui H, Gharb N (2013) Electric conductivity analysis and dielectric relaxation behavior of the hybrid polyvanadate (H3N(CH2)3NH3)[V4O10]. J Mater Res Bull 48:1978–1983. https://doi.org/10.1016/j.materresbull.2013.02.003
Ninković DB, Janjić GV, Zarić SD (2012) Crystallographic and ab initio study of pyridine stacking interactions. Local nature of hydrogen bond effect in stacking interactions. J Cryst Eng Commun 12:2113–2121. https://doi.org/10.1021/cg201389y
North ACT, Phillips DC, Mathews FS (1968) A semi-empirical method of absorption correction. Acta Cryst A24:351–359. https://doi.org/10.1107/S0567739468000707
Park BH, Hyun SJ, Moon CR, Choe BD, Lee J, Kim CY, Jo W, Noh TW (1998) Imprint failures and asymmetric electrical properties induced by thermal processes in epitaxial Bi4Ti3O12 thin films. J Appl Phys 84:4428–4435. https://doi.org/10.1063/1.368666
Parlakturk F, Altındal S, Tataroglu A, Parlak M, Agasiyev A (2008) On the profile of frequency dependent series resistance and surface states in Au/Bi4Ti3O12/SiO2/n-Si(MFIS) structures. J Microelectron Eng 85:81–88. https://doi.org/10.1016/j.mee.2007.03.012
Selvasekarapandian S, Vijaykumar M (2003) The ac impedance spectroscopy studies on LiDyO2. J Mater Chem Phys 80:29–33. https://doi.org/10.1016/s0254-0584(02)00510-2
Sheldrick GM (1997a) SHELXL-97. Program for crystal structures refinement, University of Gottingen. Electron Crystallogr. https://doi.org/10.1007/978-94-015-8971-0_18
Sheldrick GM (1997b) SHELXS-97. Program for the solution of crystal structures, University of Gottingen. Electron Crystallogr. https://doi.org/10.1007/978-94-015-8971-0_18
Spackman MA, Byrom PG (1997) A novel definition of a molecule in a crystal. J Chem Phys Lett 267:215–220. https://doi.org/10.1016/s0009-2614(97)00100-0
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. J Cryst Eng Commun 11:19–32. https://doi.org/10.1039/b818330a
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. J Cryst Eng Commun 4:378–392. https://doi.org/10.1039/b203191b
Spek AL (2003) Single-crystal structure validation with the program PLATON. J Appl Cryst 36:7–13. https://doi.org/10.1107/s0021889802022112
Weng DF, Wang BW, Wang ZM, Gao S (2011) Polymorphism of (H2mela)2[CuCl5] Cl (mela = melamine): structures, transformation and magnetic properties. Cryst Eng Commun 13:4683–4688. https://doi.org/10.1039/c1ce05143a
Wheeler SE (2012) Understanding substituent effects in noncovalent interactions involving aromatic rings. Acc Chem Res 46(4):1029–1038. https://doi.org/10.1021/ar300109n
Wolff SK, Grimwood DJ, McKinnon JJ, Turner MJ, Jayatilaka D, Spackman MA (2012) CrystalExplorer 3.1. University of Western Australia, Crawley
Acknowledgements
The authors would like to thank the members of the unit of common services, in particular Mr Tarek GARGOURI, at the University of Sfax for their useful assistance as far as the measurements of X-ray diffraction is concerned.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamdi, B., Zouari, R. & Ben Salah, A. Preparation, molecular structure, thermal properties, electrical conductivity analysis and dielectric relaxation of a new hybrid compound (NH2C5H3ClNH)2ZnBr4·H2O. Chem. Pap. 72, 2795–2811 (2018). https://doi.org/10.1007/s11696-018-0521-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0521-8