Abstract
Objective
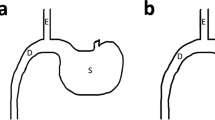
Single anastomosis duodeno-ileostomy with sleeve gastrectomy (SADI-S) is a powerful form of bariatric surgery; however, it has a high risk of malnutrition. Single anastomosis sleeve ileal (SASI) bypass with sleeve gastrectomy may be used as an alternative procedure to avoid malnutrition associated with SADI-S; however, no comparison between the two procedures has been performed.
Methods
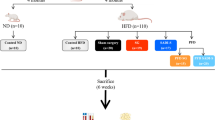
Sprague–Dawley rats with diabetes (n = 32) were divided into four groups: SADI-S (n = 8), SASI (n = 8), SG (n = 8), and SHAM (n = 8). Body weight, food intake, and fasting blood glucose were measured, and the oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were performed before and after surgery. Blood samples were collected before and after the surgery to assess the levels of glucagon-like peptide-1 (GLP-1), hemoglobin, albumin, vitamin B12, calcium, and iron.
Results
The SADI-S and SASI groups showed significantly greater weight loss and better glucose control than the SG group postoperatively. The SADI-S and SASI groups showed similar improvements in glucose control throughout the study. The SADI-S and SASI groups had significantly higher GLP-1 levels than the SG group at 6 months. The SADI-S and SASI groups presented with various degrees of deficiencies, with the SADI-S group showing a higher risk for hypoalbuminemia and iron deficiency than the SASI group.
Conclusions
The SASI procedure may be a better alternative as it has excellent bariatric and metabolic results with lower risk for hypoalbuminemia and can be easily converted into either SADI-S or SG procedures. Nevertheless, further clinical results are needed.
Graphical abstract






Similar content being viewed by others
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42.
Hanipah ZN, Schauer PR. Surgical treatment of obesity and diabetes. Gastrointest Endosc Clin N Am. 2017;27(2):191–211.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Spinos D, Skarentzos K, Esagian SM, Seymour KA, Economopoulos KP. The effectiveness of single-anastomosis duodenoileal bypass with sleeve gastrectomy/one anastomosis duodenal switch (SADI-S/OADS): an updated systematic review. Obes Surg. 2021;31(4):1790–800.
Tabesh MR, Maleklou F, Ejtehadi F, Alizadeh Z. Nutrition, physical activity, and prescription of supplements in pre- and post-bariatric surgery patients: a practical guideline. Obes Surg. 2019;29(10):3385–400.
Santoro S, Castro LC, Velhote MC, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg. 2012;256(1):104–10.
Mahdy T, Al Wahedi A, Schou C. Efficacy of single anastomosis sleeve ileal (SASI) bypass for type-2 diabetic morbid obese patients: Gastric bipartition, a novel metabolic surgery procedure: a retrospective cohort study. Int J Surg. 2016;34:28–34.
Al M, Taskin HE. Sleeve gastrectomy with transit bipartition in a series of 883 patients with mild obesity: early effectiveness and safety outcomes. Surg Endosc. 2021. https://doi.org/10.1007/s00464-021-08769-4.
Emile SH, Mahdy T, Schou C, Kramer M, Shikora S. Systematic review of the outcome of single-anastomosis sleeve ileal (SASI) bypass in treatment of morbid obesity with proportion meta-analysis of improvement in diabetes mellitus. Int J Surg. 2021 106024.
Widjaja J, Dolo PR, Zhang Q, et al. Bypassed and preserved stomach resulted in superior glucose control in Sprague-Dawley rats with streptozotocin-induced diabetes. Sci Rep. 2019;9(1):9981.
Dolo PR, Yao L, Li C, et al. Preserving duodenal-jejunal (foregut) transit does not impair glucose tolerance and diabetes remission following gastric bypass in type 2 diabetes Sprague-Dawley rat model. Obes Surg. 2018;28(5):1313–20.
Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045.
Liu P, Widjaja J, Dolo PR, et al. Comparing the anti-diabetic effect of sleeve gastrectomy with transit bipartition against sleeve gastrectomy and Roux-en-Y gastric bypass using a diabetic rodent model. Obes Surg. 2021;31(5):2203–10.
Søvik TT, Aasheim ET, Taha O, et al. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann Intern Med. 2011;155(5):281–91.
Nett P, Borbély Y, Kröll D. Micronutrient supplementation after biliopancreatic diversion with duodenal switch in the long term. Obes Surg. 2016;26(10):2469–74.
Topart P, Becouarn G, Finel JB. Is transit bipartition a better alternative to biliopancreatic diversion with duodenal switch for superobesity? Comparison of the early results of both procedures. Surg Obes Relat Dis. 2020;16(4):497–502.
Shoar S, Poliakin L, Rubenstein R, Saber AA. Single anastomosis duodeno-ileal switch (SADIS): a systematic review of efficacy and safety. Obes Surg. 2018;28(1):104–13.
Khalaf M, Hamed H. Single-Anastomosis Sleeve Ileal (SASI) Bypass: hopes and concerns after a two-year follow-up. Obes Surg. 2021;31(2):667–74.
Mahdy T, Emile SH, Madyan A, et al. Evaluation of the efficacy of single anastomosis sleeve ileal (SASI) bypass for patients with morbid obesity: a multicenter study. Obes Surg. 2020;30(3):837–45.
Sewefy AM, Saleh A. The outcomes of single anastomosis sleeve jejunal bypass as a treatment for morbid obesity (two-year follow-up). Surg Endosc. 2021;35(10):5698–704.
Osorio J, Lazzara C, Admella V, Franci-León S, Pujol-Gebellí J. Revisional Laparoscopic SADI-S vs. duodenal switch following failed primary sleeve gastrectomy: a single-center comparison of 101 consecutive cases. Obes Surg. 2021;31(8):3667–74.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741–9.
Webb DL, Abrahamsson N, Sundbom M, Hellström PM. Bariatric surgery - time to replace with GLP-1? Scand J Gastroenterol. 2017;52(6–7):635–40.
Hutch CR, Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology. 2017;158(12):4139–51.
Smith EP, Polanco G, Yaqub A, Salehi M. Altered glucose metabolism after bariatric surgery: what’s GLP-1 got to do with it? Metabolism. 2018;83:159–66.
Goh YM, Toumi Z, Date RS. Surgical cure for type 2 diabetes by foregut or hindgut operations: a myth or reality? A systematic review Surg Endosc. 2017;31(1):25–37.
Benaiges D, Más-Lorenzo A, Goday A, et al. Laparoscopic sleeve gastrectomy: more than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21(41):11804–14.
Andalib A, Aminian A. Sleeve gastrectomy and diabetes: is cure possible? Adv Surg. 2017;51(1):29–40.
Argyropoulos G. Bariatric surgery: prevalence, predictors, and mechanisms of diabetes remission. Curr Diab Rep. 2015;15(4):15.
Chu Y, Widjaja J, Hong J, Dolo PR, Zhu X, Yao L. Effect of sleeve gastrectomy on plasma thioredoxin-interacting protein (TXNIP). Obes Surg. 2021;31(11):4829–35.
Andreollo NA, Santos EF, Araújo MR, Lopes LR. Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig. 2012;25(1):49–51.
Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4(6):624–30.
Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464–74.
Funding
This study was supported by the Science and Technology Program Project of Xuzhou (KC19157).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of Informed Consent
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Statement of Animal Right
All applicable institutional and national guidelines of the People’s Republic of China for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jason Widjaja and Wei Wu contributed equally to this work.
Highlights
• The SADI-S and SASI procedures showed significantly better glucose control than the SG procedure.
• The SADI-S and SASI procedures showed similar improvements in glucose control.
• The SASI procedure has a lower risk of hypoalbuminemia and iron deficiency than the SADI-S procedure throughout the study.
Rights and permissions
About this article
Cite this article
Wu, W., Widjaja, J., Lu, S. et al. Comparison of the Outcomes of Single Anastomosis Duodeno-Ileostomy with Sleeve Gastrectomy (SADI-S), Single Anastomosis Sleeve Ileal (SASI) Bypass with Sleeve Gastrectomy, and Sleeve Gastrectomy Using a Rodent Model with Diabetes. OBES SURG 32, 1209–1215 (2022). https://doi.org/10.1007/s11695-022-05920-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-05920-5