Abstract
Purpose
Bariatric surgery may lead to metabolic bone disease.
Materials and Methods
In this cross-sectional study, we compared the prevalence of secondary hyperparathyroidism (SHPT), impact on bone mass and turnover markers, and serum leptin after Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) in 117 patients (91% female, 51% RYGB, age 41.8 ± 6.7 years, time of surgery 4.3 ± 3.4 years) at different times (1–2 years, > 2 and < 5 years and ≥ 5 years). Body composition, bone mineral density (BMD), by dual-energy X-ray absorptiometry, and bone parameters (PTH, serum calcium, 25OHD, alkaline phosphatase (AP), C-telopeptide (CTX)) were analyzed.
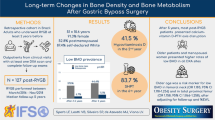
Results
Prevalence of SHPT (PTH ≥ 65 pg/ml) was 26%, RYGB > SG (18.4% vs. 7.8%, p = 0.039), despite similar 25OHD and calcium levels. Mean PTH, CTX, and AP were higher in RYGB vs. SG (61.3 ± 29.5 vs 49.5 ± 32.3 pg/ml, p = 0.001; 0.596 ± 0.24 vs. 0.463 ± 0.23 ng/ml; 123.9 ± 60.8 vs. 100.7 ± 62.0 U/l). There were 13.5% decreases in femoral neck BMD in all patients, over the study period. In the last group, the RYGB group showed greater bone loss in total body BMD (1.016 vs. 1.151 g/cm2, − 8.1%, p = 0.003) and total femur BMD (1.164 vs. 1.267 g/cm2, − 11.7%, p = 0.007). Mean leptin was lower in the RYGB vs. SG group, with no correlation with BMD in any site.
Conclusion
Our data suggest a more deleterious role of RYGB on bone remodeling up to 5 years postoperatively in comparison with SG.
Graphical abstract




Similar content being viewed by others
References
Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34.
James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–77.
Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2(3):121–33.
Viégas M, de Vasconcelos RS, Neves AP, et al. Bariatric surgery and bone metabolism: a systematic review. Arq Bras Endocrinol Metabol. 2010;54(2):158–63.
Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2(2):165–74.
Wei JH, Lee WJ, Chong K, et al. High incidence of secondary hyperparathyroidism in bariatric patients: comparing different procedures. Obes Surg. 2018;28(3):798–804.
Elaine WY, Bouxsein ML, Putman MS, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–9.
Lindeman KG, Greenblatt LB, Rourke C, et al. Longitudinal 5-year evaluation of bone density and microarchitecture after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2018;103(11):4104–12.
Tian Z, Fan XT, Li SZ, et al. Changes in bone metabolism after sleeve gastrectomy versus gastric bypass: a meta-analysis. Obes Surg. 2020;30(1):77–86.
Nakamura KM, Haglind EGC, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25(1):151–8.
English WJ, DeMaria EJ, Brethauer SA, et al. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259–63.
Costa TM da RL, Paganoto M, Radominski RB, et al. Impact of deficient nutrition in bone mass after bariatric surgery. Arq Bras Cir Dig. 2016;29(1):38–42.
Craig CL, Marshall AL, Sjöström M, et al. The International Physical Activity Questionnaire (IPAQ): a comprehensive reliability and validity study in twelve countries. Med Sci Sports Exerc. 2003;35:1381–95.
Sichieri R, Everhart JE. Validity of a brazilian food frequency questionnaire against dietary recalls and estimated energy intake. Nutr Res. 1998;18(10):1649–59.
World Health Organisation (WHO). WHO | Waist Circumference and Waist–Hip Ratio. Report of a WHO Expert Consultation. Geneva, 8–11 December 2008.
Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Lawrence Erlbaum Associates, Publishers; 1988.
Chakhtoura MT, Nakhoul NN, Shawwa K, et al. Hypovitaminosis D in bariatric surgery: a systematic review of observational studies. Metabolism. 2016;65(4):574–85.
Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30(8):1377–85.
Hewitt S, Aasheim ET, Søvik TT, et al. Relationships of serum 25-hydroxyvitamin D, ionized calcium and parathyroid hormone after obesity surgery. Clin Endocrinol (Oxf). 2018;88(3):372–9.
Carlin AM, Rao DS, Yager KM, et al. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis. 2009;5(4):444–9.
Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Switzer NJ, Marcil G, Prasad S, et al. Long-term hypovitaminosis D and secondary hyperparathyroidism outcomes of the Roux-en-Y gastric bypass: a systematic review. Obes Rev. 2017;18(5):560–6.
Diniz MDFHS, Diniz MTC, Sanches SRA, et al. Elevated serum parathormone after Roux-en-Y gastric bypass. Obes Surg. 2004;14(9):1222–6.
Vix M, Liu KH, Diana M, et al. Impact of Roux-en-Y gastric bypass versus sleeve gastrectomy on vitamin D metabolism: Short-term results from a prospective randomized clinical trial. Surg Endosc. 2014;28(3):821–6.
Salazar DA, Ferreira MJS, Neves JS, et al. Variable thresholds of vitamin D plasma levels to suppress PTH: the effect of weight and bariatric surgery. Obes Surg. 2020;30(4):1551–9.
Grethen E, Hill KM, Jones R, et al. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in Obesity. J Clin Endocrinol Metab. 2012;97(5):1655–62.
Maghrabi AH, Wolski K, Abood B, et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity. 2015;23(12):2344–8.
Jaruvongvanich V, Vantanasiri K, Upala S, et al. Changes in bone mineral density and bone metabolism after sleeve gastrectomy: a systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(8):1252–60.
Liu C, Wu D, Zhang JF, et al. Changes in bone metabolism in morbidly obese patients after bariatric surgery: a meta-analysis. Obes Surg. 2016;26(1):91–7.
Hofsø D, Hillestad TOW, Halvorsen E, et al. Bone mineral density and turnover after sleeve gastrectomy and gastric bypass: a randomized controlled trial (Oseberg). J Clin Endocrinol Metab. 2021;106(2):501–11.
Mahawar KK, Sharples AJ. Contribution of malabsorption to weight loss after Roux-en-Y gastric bypass: a systematic review. Obes Surg. Springer New York LLC; 2017; (27):2194–206.
Yu EW, Thomas BJ, Brown JK, et al. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27(1):119–24.
Vecchié A, Dallegri F, Carbone F, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6–17.
Rodríguez A, Becerril S, Méndez-Giménez L, et al. Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int J Obes (Lond). 2015;39(3):397–407.
Tothill P, Hannan WJ, Cowen S, Freeman CP. Anomalies in the measurement of changes in total-body bone mineral by dual-energy X-ray absorptiometry during weight change. J Bone Miner Res. 1997;12(11):1908–21.
Bolotin HH. A new perspective on the causal influence of soft tissue composition on DXA-measured in vivo bone mineral density. J Bone Miner Res. 1998;13(11):1739–46.
Romero-Díaz C, Duarte-Montero D, Gutiérrez-Romero SA, et al. Diabetes and bone fragility. Diabetes Ther. 2021;12(1):71–86.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) [Finance Code 001].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1. There is a high prevalence of SHPT after bariatric surgery, especially after RYGB.
2. Bariatric surgery may lead to bone loss and increased bone turnover markers.
3. After 5 years of bariatric surgery, total BMD and total femur BMD have declined more significantly in RYGB patients compared with SG.
4. There was no correlation between serum leptin and bone loss in any site after bariatric surgery.
Rights and permissions
About this article
Cite this article
de Holanda, N.C.P., Baad, V.M.A., Bezerra, L.R. et al. Secondary Hyperparathyroidism, Bone Density, and Bone Turnover After Bariatric Surgery: Differences Between Roux-en-Y Gastric Bypass and Sleeve Gastrectomy. OBES SURG 31, 5367–5375 (2021). https://doi.org/10.1007/s11695-021-05739-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05739-6