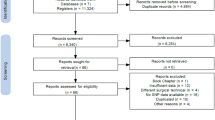
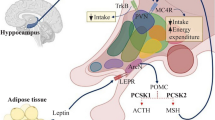
Abstract
This systematic review synthesized research evaluating the relationship between genetic predictors and weight loss after bariatric surgery. Fifty-seven studies were identified that examined single genes or genetic risk scores. Uncoupling protein (UCP) rs660339 was associated with excess weight loss after surgery in 4 of 6 studies. The most commonly assessed genes were fat mass and obesity–associated (FTO) gene (n = 10) and melanocortin-4 receptor (MC4R) (n = 14). Both were inconsistently related to weight loss. Genetic risk scores predicted weight loss in 6 of 7 studies. This evidence suggests the potential of using genetic variants and genetic risk scores to predict the amount of weight loss anticipated after bariatric surgery and identify patients who may be at risk for suboptimal weight reduction.

Similar content being viewed by others
References
Chang S-H, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–87.
Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg. 2018;153(5):427–34.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
Karlsson J, Taft C, Ryden A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes. 2007;31(8):1248–61.
Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27(4):325–51.
Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206.
Heianza Y, Qi L. Gene-diet interaction and precision nutrition in obesity. Int J Mol Sci. 2017 Apr 7;18(4):787.
Bayer S, Winkler V, Hauner H, et al. Associations between genotype–diet interactions and weight loss—a systematic review. Nutrients. 2020;12(9):2891.
Xiang L, Wu H, Pan A, et al. FTO genotype and weight loss in diet and lifestyle interventions: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103(4):1162–70.
Martínez JA, Milagro FI. Genetics of weight loss: a basis for personalized obesity management. Trends Food Sci Technol. 2015;42(2):97–115.
Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6(3):223–36.
Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12.
Sohani ZN, Meyre D, de Souza RJ, et al. Assessing the quality of published genetic association studies in meta-analyses: the quality of genetic studies (Q-Genie) tool. BMC Genet. 2015;16(1):1–8.
Bandstein M, Schultes B, Ernst B, et al. The role of FTO and vitamin D for the weight loss effect of Roux-en-Y gastric bypass surgery in obese patients. Obes Surg. 2015;25(11):2071–7.
Liou T-H, Chen H-H, Wang W, et al. ESR1, FTO, and UCP2 genes interact with bariatric surgery affecting weight loss and glycemic control in severely obese patients. Obes Surg. 2011;21(11):1758–65.
de Luis DA, Aller R, Conde R, et al. Effects of RS9939609 gene variant in FTO gene on weight loss and cardiovascular risk factors after biliopancreatic diversion surgery. J Gastrointest Surg. 2012;16(6):1194–8.
Rodrigues GK, Resende CM, Durso DF, et al. A single FTO gene variant rs9939609 is associated with body weight evolution in a multiethnic extremely obese population that underwent bariatric surgery. Nutrition. 2015;31(11-12):1344–50.
Sarzynski M, Jacobson P, Rankinen T, et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes. 2011;35(5):676–83.
Figueroa-Vega N, Jordán B, Pérez-Luque EL, et al. Effects of sleeve gastrectomy and rs9930506 FTO variants on angiopoietin/Tie-2 system in fat expansion and M1 macrophages recruitment in morbidly obese subjects. Endocrine. 2016;54(3):700–13.
Javanrouh N, Khalaj A, Guity K, et al. Presence of CC genotype for rs17773430 could affect the percentage of excess weight loss 1 year after bariatric surgery: Tehran Obesity Treatment Study (TOTS). Obes Surg. 2020;30(2):537–44.
Mirshahi UL, Still CD, Masker KK, et al. The MC4R (I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96(12):E2088–96.
Kops NL, Vivan MA, Horvath JD, et al. FABP2, LEPR223, LEP656, and FTO polymorphisms: effect on weight loss 2 years after bariatric surgery. Obes Surg. 2018;28(9):2705–11.
De Luis DA, Aller R, Sagrado MG, et al. Influence of Lys656asn polymorphism of leptin receptor gene on surgical results of biliopancreatic diversion. J Gastrointest Surg. 2010;14(5):899–903.
Chen H-H, Lee W-J, Wang W, et al. Ala55Val polymorphism on UCP2 gene predicts greater weight loss in morbidly obese patients undergoing gastric banding. Obes Surg. 2007;17(7):926–33.
Lee YC, Liew P-L, Lee W-J, et al. Prediction of successful weight reduction after laparoscopic adjustable gastric banding. Hepatogastroenterology. 2009;56(93):1222–6.
Nicoletti CF, de Oliveira APR, Brochado MJF, et al. The Ala55Val and-866G> A polymorphisms of the UCP2 gene could be biomarkers for weight loss in patients who had Roux-en-Y gastric bypass. Nutrition. 2017;33:326–30.
Sesti G, Perego L, Cardellini M, et al. Impact of common polymorphisms in candidate genes for insulin resistance and obesity on weight loss of morbidly obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. J Clin Endocrinol Metab. 2005;90(9):5064–9.
Di Renzo L, Carbonelli MG, Bianchi A, et al. Impact of the− 174 G> C IL-6 polymorphism on bioelectrical parameters in obese subjects after laparoscopic adjustable gastric banding. J Obes. 2012;2012:1–7.
Di Renzo L, Carbonelli M, Bianchi A, et al. Body composition changes after laparoscopic adjustable gastric banding: what is the role of− 174G> C interleukin-6 promoter gene polymorphism in the therapeutic strategy? Int J Obes. 2012;36(3):369–78.
Vitolo E, Santini E, Seghieri M, et al. Heterozygosity for the rs696217 SNP in the preproghrelin gene predicts weight loss after bariatric surgery in severely obese individuals. Obes Surg. 2017;27(4):961–7.
Matzko ME, Argyropoulos G, Wood GC, et al. Association of ghrelin receptor promoter polymorphisms with weight loss following Roux-en-Y gastric bypass surgery. Obes Surg. 2012;22(5):783–90.
Velázquez-Fernández D, Mercado-Celis G, Flores-Morales J, et al. Analysis of gene candidate SNP and ancestral origin associated to obesity and postoperative weight loss in a cohort of obese patients undergoing RYGB. Obes Surg. 2017;27(6):1481–92.
Hartmann IB, Fries GR, Bücker J, et al. The FKBP5 polymorphism rs1360780 is associated with lower weight loss after bariatric surgery: 26 months of follow-up. Surg Obes Relat Dis. 2016;12(8):1554–60.
Peña E, Caixàs A, Arenas C, et al. Role of the FKBP5 polymorphism rs1360780, age, sex, and type of surgery in weight loss after bariatric surgery: a follow-up study. Surg Obes Relat Dis. 2020;16(4):581–9.
Rinella ES, Still C, Shao Y, et al. Genome-wide association of single-nucleotide polymorphisms with weight loss outcomes after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2013;98(6):E1131–6.
Ciudin A, Fidilio E, Ortiz A, et al. Genetic testing to predict weight loss and diabetes remission and long-term sustainability after bariatric surgery: a pilot study. J Clin Med. 2019;8(7):964.
de Toro-Martin J, Guenard F, Tchernof A, et al. Polygenic risk score for predicting weight loss after bariatric surgery. JCI Insight. 2018;6:3(17).
Aasbrenn M, Schnurr TM, Have CT, et al. Genetic determinants of weight loss after bariatric surgery. Obes Surg. 2019;29(8):2554–61.
Katsareli E, Amerikanou C, Rouskas K, et al. A genetic risk score for the estimation of weight loss after bariatric surgery. Obes Surg. 2020;30(4):1482–90.
Nicoletti CF, Pinhel MAS, de Oliveira BAP, et al. The genetic predisposition score of seven obesity-related single nucleotide polymorphisms is associated with better metabolic outcomes after Roux-en-Y gastric bypass. J Nutrigenet Nutrigenomics. 2016;9(5-6):222–30.
Käkelä P, Jääskeläinen T, Torpström J, et al. Genetic risk score does not predict the outcome of obesity surgery. Obes Surg. 2014;24(1):128–33.
Scott F, Elahi S, Adebibe M, et al. Farnesoid X receptor-a molecular predictor of weight loss after vertical sleeve gastrectomy? Obes Sci Pract. 2019;5(3):273–80.
Balasar Ö, Çakır T, Erkal Ö, et al. The effect of rs9939609 FTO gene polymorphism on weight loss after laparoscopic sleeve gastrectomy. Surg Endosc. 2016;30(1):121–5.
Novais PFS, Weber TK, Lemke N, et al. Gene polymorphisms as a predictor of body weight loss after Roux-en-Y gastric bypass surgery among obese women. Obes Res Clin Pract. 2016;10(6):724–7.
Wang C-Y, Liu K-H, Tsai M-L, et al. FTO variants are associated with ANGPTL4 abundances and correlated with body weight reduction after bariatric surgery. Obes Res Clin Pract. 2020;14(3):257–63.
Cooiman M, Kleinendorst L, Aarts E, et al. Genetic obesity and bariatric surgery outcome in 1014 patients with morbid obesity. Obes Surg. 2020;30(2):470–7.
Potoczna N, Branson R, Kral JG, et al. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J Gastrointest Surg. 2004;8(8):971–82.
Resende CMM, Durso DF, Borges KBG, et al. The polymorphism rs17782313 near MC4R gene is related with anthropometric changes in women submitted to bariatric surgery over 60 months. Clin Nutr. 2018;37(4):1286–92.
Aslan IR, Campos GM, Calton MA, et al. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg. 2011;21(7):930–4.
Censani M, Conroy R, Deng L, et al. Weight loss after bariatric surgery in morbidly obese adolescents with MC4R mutations. Obesity (Silver Spring). 2014;22(1):225–31.
Hatoum IJ, Stylopoulos N, Vanhoose AM, et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2012;97(6):E1023–31.
Moore BS, Mirshahi UL, Yost EA, et al. Long-term weight-loss in gastric bypass patients carrying melanocortin 4 receptor variants. PLoS One. 2014;9(4):e93629.
Valette M, Poitou C, Le Beyec J, et al. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS One. 2012;7(11):e48221.
Zechner JF, Mirshahi UL, Satapati S, et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144(3):580–590. e587.
De Luis D, Pacheco D, Aller R, et al. Influence of-55CT polymorphism of UCP3 gene on surgical results of biliopancreatic diversion. Obes Surg. 2010;20(7):895–9.
de Luis DA, Sagrado MG, Izaola O, et al. Influence of Ala54Thr polymorphism of fatty acid–binding protein-2 on clinical results of biliopancreatic diversion. Nutrition. 2008;24(4):300–4.
Hulsmans M, Geeraert B, De Keyzer D, et al. Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One. 2012;7(1):e30414.
De Luis D, Pacheco D, Aller R, et al. Influence of G308A polymorphism of tumor necrosis factor alpha gene on surgical results of biliopancreatic diversion. Obes Surg. 2010;20(2):221–5.
Alexandrou A, Armeni E, Kaparos G, et al. Bsm1 vitamin D receptor polymorphism and calcium homeostasis following bariatric surgery. J Investig Surg. 2015;28(1):8–17.
Hatoum IJ, Greenawalt DM, Cotsapas C, et al. Weight loss after gastric bypass is associated with a variant at 15q26. 1. Am J Hum Genet. 2013;92(5):827–34.
Beisani M, Pappa S, Moreno P, et al. Laparoscopic sleeve gastrectomy induces molecular changes in peripheral white blood cells. Clin Nutr. 2020;39(2):592–8.
Poitou C, Lacorte J-M, Coupaye M, et al. Relationship between single nucleotide polymorphisms in leptin, IL6 and adiponectin genes and their circulating product in morbidly obese subjects before and after gastric banding surgery. Obes Surg. 2005;15(1):11–23.
Rasmussen-Torvik LJ, Baldridge AS, Pacheco JA, et al. rs4771122 predicts multiple measures of long-term weight loss after bariatric surgery. Obes Surg. 2015;25(11):2225–9.
Bandstein M, Mwinyi J, Ernst B, et al. A genetic variant in proximity to the gene LYPLAL1 is associated with lower hunger feelings and increased weight loss following Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2016;51(9):1050–5.
De Luis D, Sagrado MG, Pacheco D, et al. Effects of C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase on weight loss and cardiovascular risk factors 1 year after biliopancreatic diversion surgery. Surg Obes Relat Dis. 2010;6(5):516–20.
Leyvraz C, Verdumo C, Suter M, et al. Changes in gene expression profile in human subcutaneous adipose tissue during significant weight loss. Obes Facts. 2012;5(3):440–51.
Ruiz-Lozano T, Vidal J, De Hollanda A, et al. Evening chronotype associates with obesity in severely obese subjects: interaction with CLOCK 3111T/C. Int J Obes. 2016;40(10):1550–7.
Potoczna N, Wertli M, Steffen R, et al. G protein polymorphisms do not predict weight loss and improvement of hypertension in severely obese patients. J Gastrointest Surg. 2004;8(7):862–8.
Goergen M, Manzoni D, De Blasi V, et al. Influence of obesity-susceptibility loci (MC4R and INSIG2) on the outcome of weight loss and amelioration of co-morbidity in obese patients treated by a gastric-bypass. Bull Soc Sci Med Grand Duche Luxemb. 2011;2:7–24.
de Luis D, Calvo SG, Primo D, et al. Polymorphism rs3123554 in the cannabinoid receptor type 2 (CB2R) gene is associated with metabolic changes after biliopancreatic diversion surgery. Endocrinol Diabetes Nutr (Engl Ed). 2019;66(3):157–63.
Scuteri A, Sanna S, Chen W-M, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115.
Alalwan AA, Friedman J, Park H, et al. US national trends in bariatric surgery: a decade of study. Surgery. 2021;170:13–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Does not apply.
Conflict of Interest
Ariana M. Chao reports grants and consulting fees from WW International, Inc., outside the submitted work. Thomas A. Wadden discloses serving on advisory boards for Novo Nordisk and WW International, Inc. Robert I. Berkowitz reports receiving funding through grant support from Eisai Inc and Novo Nordisk and scientific consulting fees from WW International. The other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• FTO and MC4R are inconsistently related to weight loss after bariatric surgery.
• Genetic risk scores may be useful to predict the amount of weight loss after surgery.
• UCP was associated with excess weight loss after surgery in multiple studies.
Supplementary Information
ESM 1
(DOC 121 kb)
Rights and permissions
About this article
Cite this article
Gupta, S.R., Zhou, Y., Wadden, T.A. et al. A Systematic Review of Genetic Correlates of Weight Loss After Bariatric Surgery. OBES SURG 31, 4612–4623 (2021). https://doi.org/10.1007/s11695-021-05585-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05585-6