Abstract
Purpose
We aimed to compare the effects of Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) on postprandial glucose and lipid metabolism in addition to weight loss and fasting metabolic profile, in non-diabetic patients undergoing bariatric surgery.
Methods
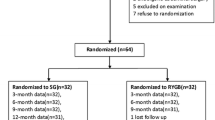
Seventy-one patients were consecutively recruited and studied preoperatively, 3 and 6 months after surgery. Of these, 28 underwent RYGB (7 males, age 38 ± 9 years, BMI 46.9 ± 5.0 kg/m2), and 43 SG (9 males, age 38 ± 9 years, BMI 50.2 ± 7.0 kg/m2). A semi-liquid mixed meal was consumed, and blood samples were taken before, and every 30 min after meal ingestion up to 180 min postprandially, for measurement of glucose, insulin, and lipids. The overall postprandial response was assessed as area under the concentration-time curve (AUC).
Results
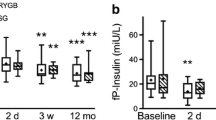
Baseline metabolic parameters were similar between RYGB and SG. Both groups experienced comparable weight loss, and a similar improvement in fasting glucose, insulin, and insulin resistance. Total and LDL cholesterol levels were lower at 6 months after RYGB compared to SG, while there was no difference in HDL cholesterol or triglycerides. Glucose AUC was lower after RYGB compared to SG at both 3 (p = 0.008) and 6 months (p = 0.016), without any difference in postprandial insulin response. Triglyceride AUC was also lower in RYGB vs. SG at 3 and 6 months (p ≤ 0.001).
Conclusions
RYGB is superior to SG in improving postprandial glycaemia and lipaemia and cholesterol profile 6 months postoperatively in non-diabetic, severely obese patients. These findings imply procedure-specific effects, such as the malabsorptive nature of RYGB, and less likely a different incretin postoperative response.



Similar content being viewed by others
References
Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82.
Ebbert JO, Elrashidi MY, Jensen MD. Managing overweight and obesity in adults to reduce cardiovascular disease risk. Curr Atheroscler Rep. 2014;16:445.
Koliaki C, Liatis S, le Roux CW, et al. The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC Endocr Disord. 2017;17:50.
Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes. 2009;33(Suppl 1):S28–32.
Chondronikola M, Harris LL, Klein S. Bariatric surgery and type 2 diabetes: are there weight loss-independent therapeutic effects of upper gastrointestinal bypass? J Intern Med. 2016;280:476–86.
Yu J, Zhou X, Li L, et al. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg. 2015;25:143–58.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–51.
Otto M, Elrefai M, Krammer J, et al. Sleeve gastrectomy and Roux-en-Y gastric bypass lead to comparable changes in body composition after adjustment for initial body mass index. Obes Surg. 2016;26:479–85.
Wang MC, Guo XH, Zhang YW, et al. Laparoscopic Roux-en-Y gastric bypass versus sleeve gastrectomy for obese patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Am Surg. 2015;81:166–71.
Woelnerhanssen B, Peterli R, Steinert RE, et al. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg Obes Relat Dis. 2011;7:561–8.
Tentolouris N, Stylianou A, Lourida E, et al. High postprandial triglyceridemia in patients with type 2 diabetes and microalbuminuria. J Lipid Res. 2007;48:218–25.
Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–31.
Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–41.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–7.
Ramon JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–22.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5.
Angelopoulos T, Kokkinos A, Liaskos C, et al. The effect of slow spaced eating on hunger and satiety in overweight and obese patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2014;2:e000013.
Kokkinos A, le Roux CW, Alexiadou K, et al. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon-like peptide-1. J Clin Endocrinol Metab. 2010;95:333–7.
Dimitriadis E, Daskalakis M, Kampa M, et al. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257:647–54.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017;27:2279–89.
Kokkinos A, Alexiadou K, Liaskos C, et al. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obes Surg. 2013;23:31–8.
Vix M, Diana M, Liu KH, et al. Evolution of glycolipid profile after sleeve gastrectomy vs. Roux-en-Y gastric bypass: results of a prospective randomized clinical trial. Obes Surg. 2013;23:613–21.
Cutolo PP, Nosso G, Vitolo G, et al. Clinical efficacy of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass in obese type 2 diabetic patients: a retrospective comparison. Obes Surg. 2012;22:1535–9.
Griffo E, Cotugno M, Nosso G, et al. Effects of sleeve gastrectomy and gastric bypass on postprandial lipid profile in obese type 2 diabetic patients: a 2-year follow-up. Obes Surg. 2016;26:1247–53.
Cunha FM, Oliveira J, Preto J, et al. The effect of bariatric surgery type on lipid profile: an age, sex, body mass index and excess weight loss matched study. Obes Surg. 2016;26:1041–7.
Benaiges D, Flores-Le-Roux JA, Pedro-Botet J, et al. Impact of restrictive (sleeve gastrectomy) vs hybrid bariatric surgery (Roux-en-Y gastric bypass) on lipid profile. Obes Surg. 2012;22:1268–75.
Benetti A, Del Puppo M, Crosignani A, et al. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malabsorptive and restrictive procedures. Diabetes Care. 2013;36:1443–7.
Pihlajamaki J, Gronlund S, Simonen M, et al. Cholesterol absorption decreases after Roux-en-Y gastric bypass but not after gastric banding. Metabolism. 2010;59:866–72.
Cowan Jr GS, Buffington CK. Significant changes in blood pressure, glucose, and lipids with gastric bypass surgery. World J Surg. 1998;22:987–92.
Frige' F, Laneri M, Veronelli A, et al. Bariatric surgery in obesity: changes of glucose and lipid metabolism correlate with changes of fat mass. Nutr Metab Cardiovasc Dis. 2009;19:198–204.
Zlabek JA, Grimm MS, Larson CJ, et al. The effect of laparoscopic gastric bypass surgery on dyslipidemia in severely obese patients. Surg Obes Relat Dis. 2005;1:537–42.
Garcia-Marirrodriga I, Amaya-Romero C, Ruiz-Diaz GP, et al. Evolution of lipid profiles after bariatric surgery. Obes Surg. 2012;22:609–16.
Pohle-Krauza RJ, McCarroll ML, Pasini DD, et al. Age and gender exert differential effects on blood lipids in patients after LAGB and LRYGB. Surg Obes Relat Dis. 2011;7:170–5.
Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–8.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671–7.
Kumar R, Lieske JC, Collazo-Clavell ML, et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149:654–61.
Stefater MA, Sandoval DA, Chambers AP, et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141:939–949.e1-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic Supplementary Material
Supplementary Figure 1
Postprandial time course of insulin during the mixed meal test (a) at baseline, (b) 3 months and (c) 6 months after RYGB and SG. (d) Total area under the curve (AUC) for postprandial insulin (0-180 minutes). Data are presented as mean±SEM. Panels a-c: Black triangles represent RYGB and grey squares represent SG group; the level of statistical significance was defined as p <0.05/7=0.007 (Bonferroni correction for 7 time points) Panel d: dark grey columns represent RYGB and light grey columns represent SG; the level of statistical significance was defined as p <0.05/3=0.017 (Bonferroni correction for 3 time points). p=ns (non-significant) for RYGB vs. SG at any time point. AUC: area under the curve; RYGB: Roux-en-Y Gastric Bypass; SG: Sleeve Gastrectomy (DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Liaskos, C., Koliaki, C., Alexiadou, K. et al. Roux-en-Y Gastric Bypass Is More Effective than Sleeve Gastrectomy in Improving Postprandial Glycaemia and Lipaemia in Non-diabetic Morbidly Obese Patients: a Short-term Follow-up Analysis. OBES SURG 28, 3997–4005 (2018). https://doi.org/10.1007/s11695-018-3454-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3454-y