Abstract
Background
The unique effects of gastric resection after vertical sleeve gastrectomy (VSG) on type 2 diabetes mellitus remain unclear. This work aimed to investigate the effects of VSG on gastric leptin expression and intestinal glucose absorption in high-fat diet-induced obesity.
Methods
Male C57BL/6J mice were fed a high-fat diet (HFD) to induce obesity. HFD mice were randomized into VSG and sham-operation groups, and the relevant parameters were measured at 8 weeks postoperation.
Results
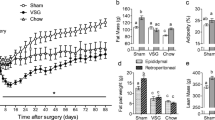
Higher gastric leptin expression and increased intestinal glucose transport were observed in the HFD mice. Furthermore, VSG reduced gastric leptin expression and the intestinal absorption of alimentary glucose. Both exogenous leptin replenishment during the oral glucose tolerance test (OGTT) and the addition of leptin into the everted isolated jejunum loops in vitro restored the glucose transport capacity in VSG-operated mice, and this effect was abolished when the glucose transporter GLUT2 was blocked with phloretin. Moreover, phloretin almost completely suppressed glucose transport in the HFD mice. Intestinal immunohistochemistry in the obese mice showed increased GLUT2 and diminished sodium glucose co-transporter 1 (SGLT-1) in the apical membrane of enterocytes. Decreased GLUT2 and enhanced SGLT1 were observed following VSG. VSG also reduced the phosphorylation status of protein kinase C isoenzyme β II (PKCβ II) in the jejunum, which was stimulated by the combination of leptin and glucose.
Conclusion
Our data demonstrated that the decreased secretion of gastric leptin in VSG results in a decrease in intestinal glucose absorption via modulation of GLUT2 translocation.





Similar content being viewed by others
References
Brito JP, Montori VM, Davis AM. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. JAMA. 2017;317(6):635–6.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–32.
Cavin JB, Bado A, Le Gall M. Intestinal adaptations after bariatric surgery: consequences on glucose homeostasis. Trends Endocrinol Metab. 2017;28(5):354–64.
Rosenthal RJ, Diaz AA, Arvidsson D, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19.
Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J. 2014;38(6):406–15.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Bueter M, Lowenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138(5):1845–53.
le Roux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50–6.
Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science (New York, NY). 2013;341(6144):406–10.
Cavin JB, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, Cluzeaud F, Gillard L, Hourseau M, Mikail N, Ribeiro-Parenti L, Kapel N, Marmuse JP, Bado A, le Gall M Differences in alimentary glucose absorption and intestinal disposal of blood glucose after roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology 2016, 150(2):454–64.e9, 464.e9.
Mumphrey MB, Hao Z, Townsend RL, et al. Sleeve gastrectomy does not cause hypertrophy and reprogramming of intestinal glucose metabolism in rats. Obes Surg. 2015;25(8):1468–73.
Myers Jr MG, Olson DP. Central nervous system control of metabolism. Nature. 2012;491(7424):357–63.
Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394(6695):790–3.
Sobhani I, Bado A, Vissuzaine C, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47(2):178–83.
Ducroc R, Guilmeau S, Akasbi K, et al. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes. 2005;54(2):348–54.
Sakar Y, Nazaret C, Letteron P, et al. Positive regulatory control loop between gut leptin and intestinal GLUT2/GLUT5 transporters links to hepatic metabolic functions in rodents. PLoS One. 2009;4(11):e7935.
Barrenetxe J, Villaro AC, Guembe L, et al. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut. 2002;50(6):797–802.
Buyse M, Sitaraman SV, Liu X, et al. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem. 2002;277(31):28182–90.
Buyse M, Berlioz F, Guilmeau S, et al. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001;108(10):1483–94.
Tavernier A, Cavin JB, Le Gall M, et al. Intestinal deletion of leptin signaling alters activity of nutrient transporters and delayed the onset of obesity in mice. FASEB J. 2014;28(9):4100–10.
Andrikopoulos S, Blair AR, Deluca N, et al. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–32.
Xia Z, Wang G, Li H, et al. Influence of bariatric surgery on the expression of nesfatin-1 in rats with type 2 diabetes mellitus. Curr Pharm Des. 2015;21(11):1464–71.
Guilmeau S, Buyse M, Tsocas A, et al. Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes. 2003;52(7):1664–72.
Du JP, Wang G, Hu CJ, et al. IFN-gamma secretion in gut of Ob/Ob mice after vertical sleeve gastrectomy and its function in weight loss mechanism. J Huazhong Univ Sci Technolog Med Sci. 2016;36(3):377–82.
Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531(Pt 3):585–95.
Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350(Pt 1):155–62.
Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol. 2012;45(1):1–16.
Le Beyec J, Pelletier AL, Arapis K, et al. Overexpression of gastric leptin precedes adipocyte leptin during high-fat diet and is linked to 5HT-containing enterochromaffin cells. Int J Obes (2005). 2014;38(10):1357–64.
Jimenez A, Ceriello A, Casamitjana R, et al. Remission of type 2 diabetes after roux-en-Y gastric bypass or sleeve gastrectomy is associated with a distinct glycemic profile. Ann Surg. 2015;261(2):316–22.
Le Gall M, Tobin V, Stolarczyk E, et al. Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism. J Cell Physiol. 2007;213(3):834–43.
Lehmann A, Hornby PJ. Intestinal SGLT1 in metabolic health and disease. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G887–98.
Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54(10):3056–62.
Ait-Omar A, Monteiro-Sepulveda M, Poitou C, et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and Ob/Ob and high fat-fed mice. Diabetes. 2011;60(10):2598–607.
Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–6.
Woelnerhanssen B, Peterli R, Steinert RE, et al. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg Obes Relat Dis. 2011;7(5):561–8.
Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):E424–32.
Valderas JP, Irribarra V, Rubio L, et al. Effects of sleeve gastrectomy and medical treatment for obesity on glucagon-like peptide 1 levels and glucose homeostasis in non-diabetic subjects. Obes Surg. 2011;21(7):902–9.
Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–9.
Jimenez A, Mari A, Casamitjana R, et al. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes. 2014;63(10):3372–7.
Ye J, Hao Z, Mumphrey MB, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306(5):R352–62.
Wilson-Perez HE, Chambers AP, Ryan KK, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–5.
Rodriguez A, Becerril S, Valenti V, et al. Short-term effects of sleeve gastrectomy and caloric restriction on blood pressure in diet-induced obese rats. Obes Surg. 2012;22(9):1481–90.
Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring, Md). 2014;22(2):390–400.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring, Md). 2009;17(9):1671–7.
Belgaumkar AP, Vincent RP, Carswell KA, et al. Changes in bile acid profile after laparoscopic sleeve gastrectomy are associated with improvements in metabolic profile and fatty liver disease. Obes Surg. 2016;26(6):1195–202.
Ma Y, Huang Y, Yan L, et al. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm Res. 2013;30(5):1447–57.
Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–65.
Liang CP, Tall AR. Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in Ob/Ob mouse liver. J Biol Chem. 2001;276(52):49066–76.
Funding
This study was supported by National Key Basic Research Program of China (No. 2015CB5540007); National Natural Science Foundation of China (No. 81472740, 81200276, and 81700488); Natural Science Foundation of Hubei Province of China (No. 2014CFA060 and 2015CFB710); Research Fund of Public Welfare in Health Industry, Health and Family Plan Committee of China (No. 201402015); Natural Science Foundation of Huazhong University of Science and Technology (No. 5001530030), and Health and Family Planning Youth Project Foundation of Hubei Province, China (No. WJ2015Q001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal studies (including the mice euthanasia procedure) were conducted in compliance with the regulations and guidelines of Tongji Medicine College institutional animal care committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
A Statement of Animal Rights/Ethical Approval
All procedures in this study were approved by the Ethics Committee for Animal Research of Tongji Medicine College.
Additional information
Jinpeng Du and Chaojie Hu are co-first authors.
Electronic supplementary material
Table S1
(DOCX 12 kb)
Figure S1
Study flow chart. (PNG 85 kb)
High Resolution Image
(TIF 494 kb)
Figure S2
Images of VSG before (A) and after (B) stomach resection along the greater curvature. (PNG 403 kb)
High Resolution Image
(TIF 1187 kb)
Figure S3
Photographs of surgical implantation of an outflow catheter 1 cm below the ligament of Treitz for the collection of duodenal juice. (PNG 488 kb)
High Resolution Image
(TIF 1319 kb)
Figure S4
Glucose transport in vitro using everted isolated jejunum loops from ND and HFD mice after a 16-week feeding. (A) Time course of mucosal-to-serosal glucose transport across the jejunum of the ND and HFD mice with or without phloretin in association with 30 mM glucose. (B) Cumulative glucose transport of jejunum segments at 60 min. Values are expressed as the means ± SEM, ***P < 0.001 vs. HFD, based on one-way analysis of variance with Bonferroni correction for multiple comparisons, n = 6 per group. (PNG 111 kb)
High Resolution Image
(TIF 493 kb)
Figure S5
Body weight changes (A), OGTT results (B) and glucose transporter immunohistochemistry (C) of pair-fed (PF) mice, ND mice, sham mice and VSG mice at 8 weeks after surgery. Values are the means ± SEM, n = 6 per group. (PNG 1588 kb)
High Resolution Image
(TIF 7462 kb)
Rights and permissions
About this article
Cite this article
Du, J., Hu, C., Bai, J. et al. Intestinal Glucose Absorption Was Reduced by Vertical Sleeve Gastrectomy via Decreased Gastric Leptin Secretion. OBES SURG 28, 3851–3861 (2018). https://doi.org/10.1007/s11695-018-3351-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3351-4