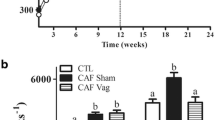
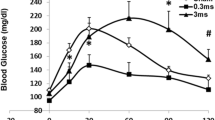
Abstract
The current treatment for obesity-related type 2 diabetes is not able to achieve sufficient metabolic control. New remission prospects have been offered through bariatric surgery and other interventional therapies. The aim of the study is to illustrate the mechanism by which such therapies affect the autonomic system, in particular the afferent vagal activity. The first and most important terminal of this activity is the brainstem vagal nucleus tractus solitarius. Its function, on which the vagal efferent inputs that control the splanchnic organs depend, is conditioned by the level of synaptic transmission within it. In conclusion, on the basis of such a view, a selective pharmacological modulation of such transmission as the target for future medical treatment of obesity and related type 2 diabetes is proposed.




Similar content being viewed by others
References
Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–9.
Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304.
Scott JD, Johnson BL, Blackhurst DW, et al. Does bariatric surgery reduce the risk of major cardiovascular events? A retrospective cohort study of morbidly obese surgical patients. Surg Obes Relat Dis. 2013;9(1):32–9.
Salinari S, Bertuzzi A, Asnaghi S, et al. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32(3):375–80.
Côté CD, Zadeh-Tahmasebi M, Rasmussen BA, et al. Hormonal signaling in the gut. J Biol Chem. 2014;289(17):11642–9.
Canales BK, Gonzalez RD. Kidney stone risk following Roux-en-Y gastric bypass surgery. Transl Urol Androl. 2014;3(3):242–9.
Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152–64.
Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol. 2014;28(4):741–9.
Hussain SS, Bloom SR. The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes. 2013;37(5):625–33.
Stefater MA, Wilson-Pérez HE, Chambers AP, et al. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33(4):595–622.
Lutz TA, Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. Am J Physiol Regul Integr Comp Physiol. 2014;307(11):R1275–91.
Blasi C. Can diabetes heal?—from observations to perspectives. Curr Diabetes Rev. 2016;12(3):184–98.
Berthoud HR, Zheng H, Shin AC. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Ann N Y Acad Sci. 2012;1264:36–48.
Bach EC, Halmos KC, Smith BN. Enhanced NMDA receptor-mediated modulation of excitatory neurotransmission in the dorsal vagal complex of streptozotocin-treated, chronically hyperglycemic mice. PLoS One. 2015;10(3):e0121022.
Zhao K, Ao Y, Harper RM, et al. Food-intake dysregulation in type 2 diabetic goto-kakizaki rats: hypothesized role of dysfunctional brainstem thyrotropin-releasing hormone and impaired vagal output. Neuroscience. 2013;247:43–54.
Browning KR, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci. 2011;161(1–2):6–13.
Powley TL. Vagal circuitry mediating cephalic-phase responses to food. Appetite. 2000;34(2):184–8.
Shin AC, Berthoud HR. Obesity surgery: happy with less or eternally hungry? Trends Endocrinol Metab. 2013;24(2):101–8.
de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;9.
Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr Opin Clin Nutr Metab Care. 2004;7(4):471–8.
Avetisyan M, Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest. 2015;125(3):899–907.
Bohórquez DV, Liddle RA. The gut connectome: making sense of what you eat. J Clin Invest. 2015;125(3):888–90.
Vermeulen W, De Man JG, Pelckmans PA, et al. Neuroanatomy of lower gastrointestinal pain disorders. World J Gastroenterol. 2014;20(4):1005–20.
Rasoamanana R, Darcel N, Fromentin G, et al. Nutrient sensing and signaling by the gut. Proc Nutr Soc. 2012;71(4):446–55.
Punjabi M, Arnold M, Geary N, et al. Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav. 2011;105(1):71–6.
Hayes MR, Kanoski SE, De Jonghe BC, et al. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1479–85.
Burcelin R. The gut-brain axis: a major glucoregulatory player. Diabetes Metab. 2010;36(Suppl 3):S54–8.
Covasa M. CCK- and leptin-induced vagal afferent activation: a model for organ-specific endocrine modulation of visceral sensory information. Am J Physiol Regul Integr Comp Physiol. 2006;290(6):R1542–3.
Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest. 2015;125(3):908–17.
Ritter RC. A tale of two endings: modulation of satiation by NMDA receptors on or near central and peripheral vagal afferent terminals. Physiol Behav. 2011;105(1):94–9.
Haines DE. Neuroanatomy. An atlas of structures, sections, and systems. Lippincott Williams & Wilkins; 2004.
Schneeberger M, Gomis R, Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J Endocrinol. 2014;220(2):T25–46.
Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol. 2013;591(9):2357–72.
Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97(6):1611–26.
Browning KN, Babic T, Toti L, et al. Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor. J Physiol. 2014;592(20):4591–605.
Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82(2):430–43.
Gerrow K, Triller A. Synaptic stability and plasticity in a floating world. Curr Opin Neurobiol. 2010;20(5):631–9.
Shouval HZ, Castellani GC, Blais BS, et al. Converging evidence for a simplified biophysical model of synaptic plasticity. Biol Cybern. 2002;87(5–6):383–91.
Wu SW, Fenwick AJ, Peters JH. Channeling satiation: a primer on the role of TRP channels in the control of glutamate release from vagal afferent neurons. Physiol Behav. 2014;136:179–84.
Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S Suppl):1007S–15S.
Baude A, Strube C, Tell F, et al. Glutamatergic neurotransmission in the nucleus tractus solitary: structural and functional characteristics. J Chem Neuroanat. 2009;38(3):145–53.
Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol. 2010;103(2):904–14.
Davis SF, Derbenev AV, Williams KW, et al. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017(1–2):208–17.
Travagli RA, Rogers RC. Receptors and transmission in the brain-gut axis: potential for novel therapies. V. Fast and slow extrinsic modulation of dorsal vagal complex circuits. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G595–601.
Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108(Suppl 4a):79S–86S.
Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4(4):1339–68.
Blake CB, Smith BN. cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R711–20.
Ballsmider LA, Vaughn AC, David M, et al. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015;2015:601985.
Kentish SJ, Page AJ. The role of gastrointestinal vagal afferent fibres in obesity. J Physiol. 2015;593(4):775–86.
Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex-linking immunity and metabolism. Nat Rev Endocrinol. 2012;8(12):743–54.
Daly DM, Park SJ, Valinsky WC, et al. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589(PT 11):2857–70.
Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol. 2014;592(14):2927–41.
de Lartigue G, Ronveaux CC, Raybould HE. Vagal plasticity the key to obesity. Molecular Metabolism. 2014;3(9):855–6.
Furnes MW, Zhao CM, Chen D. Development of obesity is associated with increased calories per meal rather than per day. A study of high-fat diet-induced obesity in young rats. Obes Surg. 2009;19(10):1430–8.
Dockray GJ, Burdyga G. Plasticity in vagal afferent neurons during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf). 2011;201(3):313–21.
Page AJ. Vagal afferent dysfunction in obesity: cause or effect. J Physiol. 2016;594(1):5–6.
Kral JG, Paez W, Wolfe BM. Vagal nerve function in obesity: therapeutic implications. World J Surg. 2009;33(10):1995–2006.
Stearns AT, Balakrishnan A, Radmanesh A, et al. Relative contributions of afferent vagal fibers to resistance to diet-induced obesity. Dig Dis Sci. 2012;57(5):1281–90.
Leung FW. Capsaicin as an anti-obesity drug. Prog Drug Res. 2014;68:171–9.
Westerterp-Plantenga MS, Smeets A, Lejeune MP. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int J Obes. 2005;29(6):682–8.
Faris PL, Kim SW, Meller WH, et al. Effect of decreasing afferent vagal activity with ondansetron on symptoms of bulimia nervosa: a randomised, double-blind trial. Lancet. 2000;355(9206):792–7.
Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45(1):41–50.
Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol. 2008;164(1–2):105–11.
Bonham AC, Chen CY, Sekizawa S, et al. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol. 2006;101(1):322–7.
Val-Laillet D, Aarts E, Weber B, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31.
Donovan MJ, Paulino G, Raybould HE. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav. 2007;92(5):969–74.
Covasa M, Ritter RC. Adaptation to high-fat diet reduces inhibition of gastric emptying by CCK and intestinal oleate. Am J Physiol Regul Integr Comp Physiol. 2000;278(1):R166–70.
Zsombok A, Bhaskaran MD, Gao H, et al. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci. 2011;31(39):14024–31.
Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26.
Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34.
Ter Horst GJ, de Boer P, Luiten PG, et al. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31(3):785–97.
Jean A. Le noyau du faisceau solitaire: aspects neuroanatomiques, neurochimiques et fonctionnels. Arch Int Physiol Bioch Biophys. 1991;99:A3–A52.
Van den Oever MC, Spijker S, Smit AB. The synaptic pathology of drug addiction. Adv Exp Med Biol. 2012;970:469–91.
Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med. 2009;361(3):302–3.
Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76 Pt B:276–86.
Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974–86.
Peters JH, Gallaher ZR, et al. Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy. J Comp Neurol. 2013;521(15):3584–99.
Gallaher ZR, Ryu V, Herzog T, et al. Changes in microglial activation within the hindbrain, nodose ganglia, and the spinal cord following subdiaphragmatic vagotomy. Neurosci Lett. 2012;513(1):31–6.
Andreelli F, Amouyal C, Magnan C, et al. What can bariatric surgery teach us about the pathophysiology of type 2 diabetes? Diabetes Metab. 2009;35(6 Pt 2):499–507.
Karim R, Chaudhri P. Behavioral addictions: an overview. J Psychoactive Drugs. 2012;44(1):5–17.
Sussman S, Lisha N, Griffiths M. Prevalence of the addictions: a problem of the majority or the minority? Eval Health Prof. 2011;34(1):3–56.
Pang ZP, Han W. Regulation of synaptic functions in central nervous system by endocrine hormones and the maintenance of energy homoeostasis. Biosci Rep. 2012;32(5):423–32.
Dunn JP, Cowan RL, Volkow ND, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res. 2010;1350:123–30.
Breen DM, Rasmussen BA, Côté CD, et al. Nutrient-sensing mechanisms in the gut as therapeutic targets for diabetes. Diabetes. 2013;62(9):3005–13.
Kullmann S, Heni M, Veit R, et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015;38(6):1044–50.
Manning S, Pucci A, Batterham RL. Roux-en-Y gastric bypass: effects on feeding behavior and underlying mechanisms. J Clin Invest. 2015;125(3):939–48.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5.
Larder R, O’Rahilly S. Shedding pounds after going under the knife: guts over glory-why diets fail. Nat Med. 2012;18(5):666–7.
Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10(10):575–84.
Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R15–27.
Bueter M, Löwenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138(5):1845–53.
Schultes B, Ernst B, Wilms B, et al. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92(2):277–83.
Flancbaum L, Choban PS, Bradley LR, et al. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery. 1997;122(5):943–9.
Delin CR, Watts JM, Saebel JL, et al. Eating behavior and the experience of hunger following gastric bypass surgery for morbid obesity. Obes Surg. 1997;7(5):405–13.
Chambers AP, Wilson-Perez HE, McGrath S, et al. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab. 2012;303(8):E1076–84.
Steele KE, Prokopowicz GP, Schweitzer MA, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 2010;20(3):369–74.
Hao Z, Townsend RL, Mumphrey MB, et al. Vagal innervation of the intestine contributes to weight loss after Roux-e-Y gastric bypass surgery in rats. Obes Surg. 2014;24(12):2145–51.
Gautron L, Zechner J, Aguirre V. Vagal innervation patterns following roux-en-Y gastric bypass in the mouse. Int J Obes. 2013;37(12):1603–7.
Yamazaki H, Tsuboya T, Tsuji K, et al. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes mellitus. Diabetes Care. 2015;38(9):1673–9.
Yki-Järvinen H. Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig Dis. 2010;28(1):203–9.
Immonen H, Hannukainen JC, Iozzo P, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol. 2014;60(2):377–83.
Quercia I, Dutia R, Kotler DP, et al. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014;40(2):87–94.
He B, Piao D, Yu C, et al. Amelioration in hepatic insulin sensitivity by reduced hepatic lipid accumulation at short-term after Roux-en-Y gastric bypass surgery in type 2 diabetic rats. Obes Surg. 2013;23(12):2033–41.
Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia. 2008;51(10):1781–9.
Yue JT, Abraham MA, LaPierre MP, et al. A fatty acid-dependent hypothalamic-DVC neurocircuitry that regulates hepatic secretion of triglyceride-rich lipoproteins. Nat Commun. 2015;6:5970.
Lam CK, Chari M, Rutter GA, et al. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes. 2011;60(1):107–13.
Cohen R, le Roux CW, Papamargaritis D, et al. Role of proximal gut exclusion from food on glucose homeostasis in patients with type 2 diabetes. Diabet Med. 2013;30(12):1482–6.
de Jonge C, Rensen SS, Verdam FJ, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg. 2013;23(9):1354–60.
Fractyl Labs. Positive clinical data for first procedural therapy to treat type 2 diabetes. In: Abstracts from the 19th world congress of the international federation for the surgery of obesity & metabolic disorders (IFSO). Obes Surg. 2014;24(8):1170.
Kamvissi V, Salerno A, Bornstein SR, et al. Incretins or anti-incretins? A new model for the “entero-pancreatic axis”. Horm Metab Res. 2015;47(1):84–7.
Farré R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol. 2013;108(5):698–706.
Berthoud HR, Kressel M, Raybould HE, et al. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl). 1995;191(3):203–12.
Kressel M, Berthoud HR, Neuhuber WL. Vagal innervation of the rat pylorus: an anterograde tracing study using carbocyanine dyes and laser scanning confocal microscopy. Cell Tissue Res. 1994;275(1):109–23.
Holst JJ. Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Curr Opin Pharmacol. 2013;13(6):983–8.
Daniel EE, Wiebe GE. Transmission of reflexes arising on both sides of the gastroduodenal junction. Am J Phys. 1966;211(3):634–42.
Shikora S, Toouli J, Herrera MF, et al. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J Obes. 2013;2013:245683.
Val-Laillet D, Biraben A, Randuineau G, et al. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in obese adult minipigs. Appetite. 2010;55(2):245–52.
Smith DK, Sarfeh J, Howard L. Truncal vagotomy in hypothalamic obesity. Lancet. 1983;1(8337):1330–1.
Lebovitz HE, Ludvik B, Yaniv I, et al. Gutterman DD; Metacure Investigators. Treatment of patients with obese type 2 diabetes with Tantalus-DIAMOND® gastric electrical stimulation: normal triglycerides predict durable effects for at least 3 years. Horm Metab Res. 2015;47(6):456–62.
Peles S, Petersen J, Aviv R, et al. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285(3):G577–85.
Goldman JM, Wheeler MF. Remission of diabetes after irradiation of head and neck. Diabetes Care. 1987;10(1):137–8.
Raheja BS, Motwani BT, Mehta AR, et al. Remission of NIDDM after irradiation of metastatic cervical lymph nodes. Diabetes Care. 1986;9(1):101–3.
Rex D, Duckworth WC. Remission of overt diabetes mellitus after removal of an oral epidermoid carcinoma. Am J Med Sci. 1984;287(3):43–5.
Ricard D, Soussain C, Psimaras D. Neurotoxicity of the CNS: diagnosis, treatment and prevention. Rev Neurol (Paris). 2011;167(10):737–45.
Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222(4):560–77.
Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069–74.
Torii K, Uneyama H, Nakamura E. Physiological roles of dietary glutamate signaling via gut-brain axis due to efficient digestion and absorption. J Gastroenterol. 2013;48(4):442–51.
Niijima A. Effect of umami taste stimulations on vagal efferent activity in the rat. Brain Res Bull. 1991;27(3–4):393–6.
Schier LA, Davidson TL, Powley TL. Rapid stimulus-bound suppression of intake in response to an intraduodenal nonnutritive sweetener after training with nutritive sugars predicting malaise. Am J Physiol Regul Integr Comp Physiol. 2012;302(11):R1351–63.
Freeman AJ, Cunningham KT, Tyers MB. Selectivity of 5-HT3 antagonists and anti-emetic mechanisms of action. Anti-Cancer Drugs. 1992;3(2):79–85.
Wood PL. The NMDA receptor complex: a long and winding road to therapeutics. IDrugs. 2005;8(3):229–35.
Vyklicky V, Korinek M, Smejkalova T, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–203.
Tomek SE, LaCrosse AL, Nemirovsky NE, et al. NMDA receptor modulators in the treatment of drug addiction. Pharmaceuticals. 2013;6(2):251–68.
Acknowledgments
The author wishes to thank Professor Domenico Andreani, President of DEM Foundation, for his constant encouragement in the study of the subject of the present review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Informed Consent
Does not apply.
Human and Animal Rights
Does not apply.
Rights and permissions
About this article
Cite this article
Blasi, C. The Role of the Vagal Nucleus Tractus Solitarius in the Therapeutic Effects of Obesity Surgery and Other Interventional Therapies on Type 2 Diabetes. OBES SURG 26, 3045–3057 (2016). https://doi.org/10.1007/s11695-016-2419-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2419-2