Abstract
Background
Mitochondrial dysfunction in adipose tissue has been implicated as a pathogenic step in the development of type 2 diabetes mellitus (T2DM). In adipose tissue, chronic nutrient overload results in mitochondria driven increased reactive oxygen species (ROS) leading to carbonylation of proteins that impair mitochondrial function and downregulation of key genes linked to mitochondrial biogenesis. In patients with T2DM, Roux-en-Y gastric bypass (RYGB) surgery leads to improvements in glycemic profile prior to significant weight loss. Consequently, we hypothesized that improved glycemia early after RYGB would be paralleled by decreased protein carbonylation and increased expression of genes related to mitochondrial biogenesis in adipose tissue.
Methods
To evaluate this hypothesis, 16 obese individuals were studied before and 7–8 days following RYGB and adjustable gastric banding (AGB). Subcutaneous adipose tissue was obtained pre- and post-bariatric surgery as well as from eight healthy, non-obese individual controls.
Results
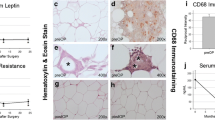
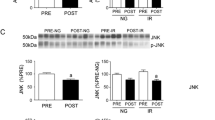
Prior to surgery, adipose tissue expression of PGC1α, NRF1, Cyt C, and eNOS (but not Tfam) showed significantly lower expression in the obese bariatric surgery group when compared to lean controls (p < 0.05). Following RYGB, but not after AGB, patients showed significant decrease in HOMA-IR, reduction in adipose protein carbonylation, and increased expression of genes linked to mitochondrial biogenesis.
Conclusions
These results suggest that rapid reduction in protein carbonylation and increased mitochondrial biogenesis may explain postoperative metabolic improvements following RYGB.






Similar content being viewed by others
References
Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–9. doi:10.1001/jama.2013.5835.
Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–42. Epub 2010 Apr 5.
Dunn JP, Abumurad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–42.
Malandrucco I, Pasqualetti P, Giordani I, et al. Very-low-calorie diet: a quick therapeutic tool to improve b cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr. 2012;95:609–13.
Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol. 2014;99(9):1168–78. doi:10.1113/expphysiol.2014.081414. Epub 2014 Aug 15.
De Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54(6):945–55.
Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep. 2008;8(3):173–8.
Viera VJ, Valentine RJ. Mitochondrial biogenesis in adipose tissue: can exercise make fat cells ‘fit’? J Physiol. 2009;587(14):3427–8.
Mootha VK, Lindgren CM, Eriksson K, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–73.
Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–71.
Elizalde M, Rydén M, van Harmelen V, et al. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J Lipid Res. 2000;41(8):1244–51.
Hickner RC, Kemeny G, Stallings HW, et al. Relationship between body composition and skeletal muscle eNOS. Int J Obes. 2006;30(2):308–12.
Curtis JM, Grimsrud PA, Wright WS, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132–42.
Demozay D, Mas J, Rocchi S, et al. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Diabetes. 2008;57(5):1216–26.
Tormos KV, Anso E, Hamanaka RB, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14(4):537–44. doi:10.1016/j.cmet.2011.08.007.
Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–8.
Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23(4):281–305.
Grimsrud PA, Picklo MJ, Griffin TJ, et al. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6(4):624–37.
Meany DL, Xie H, Thompson LV, et al. Identification of carbonylated proteins from enriched rat skeletal muscle mitochondria using affinity chromatography-stable isotope labeling and tandem mass spectrometry. Proteomics. 2007;7(7):1150–63.
Chattopadhyay M, Guhathakurta I, Behera P, et al. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism. 2011;60(12):1702–10. doi:10.1016/j.metabol.2011.04.015. Epub 2011 Jun 12.
Dahlman I, Forsgren M, Sjogren A, et al. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-α. Diabetes. 2006;55(6):1792–9.
Valerio A, Cardile A, Cozzi V, et al. TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116(10):2791–8. Epub 2006 Sep 14.
Hotamisligil GS, Johnson RS, Distel RJ, et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–9.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Bonora E, Targger G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degree of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63.
Ikramuddin S, Kendrick ML, Kellogg TA, et al. Open and laparoscopic Roux-en-Y gastric bypass: our techniques. J Gastrointest Surg. 2007;11(2):217–28.
Beitner M, Kurian MS. Laparoscopic adjustable gastric banding. Abdom Imaging. 2012.
Frohnert BI, Sinaiko AR, Serrot FJ, et al. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring). 2011;19(9):1735–41.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256.e5.
Dankel SN, Staalesen V, Bjørndal B, et al. Tissue-specific effects of bariatric surgery including mitochondrial function. J Obes. 2011;2011:435245.
Wickremesekera K, Miller G, DeSilva Naotunne T, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–81.
Lima MM, Pareja JC, Alegre SM, et al. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(8):3871–5.
Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. J Am Med Assoc. 2008;299(3):316–23.
Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14(1):15–23.
Tammy L, Kindel, Paulo JF, et al. Bypassing the duodenum does not improve insulin resistance associated with diet-induced obesity in rodents. Obesity. 2011;19(2):380–7.
Newsholme P, Gaudel C, Krause M. Mitochondria and diabetes. An intriguing pathogenetic role. Adv Exp Med Biol. 2012;942:235–47.
Brands M, Verhoeven AJ, Serlie MJ. Role of mitochondrial function in insulin resistance. Adv Exp Med Biol. 2012;942:215–34.
Martins AR, Nachbar RT, Gorjao R, et al. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis. 2012;11:30.
Pagel-Langenickel I, Bao J, Pang L, et al. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010;31–1:25–51.
Dubé JJ, Amati F, Toledo GS, et al. Effects of weight loss and exercise on insulin resistance and intramyocellular triacylglycerol, diacylglyceroland ceramide. Diabetologia. 2011;54:1147–56.
Dankel SN, Fadnes DJ, Stavrum AK, et al. Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss. PLoS One. 2010;5–6:e11033.
Hernandez-Alvarez MI, Chiellini C, Manco M, et al. Genes involved in mitochondrial biogenesis/function are induced in response to bilio-pancreatic diversion in morbidly obese individuals with normal glucose tolerance but not in type 2 diabetic patients. Diabetologia. 2009;52–8:1618–27.
Xu H, Hertzel AV, Steen KA, et al. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Mol Cell Biol. 2015;35(6):1055–65. doi:10.1128/MCB.01122-14. Epub 2015 Jan 12.
Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. Review.
Acknowledgments
This work was supported by the American Diabetes Association (ADA 7-11-ST-01), The Minnesota Obesity Center (NIH DK050456) and NIH DK084669. We thank the members of the Bernlohr laboratory for helpful comments and suggestions during the preparation of this manuscript. We also thank transplant surgeons Drs Ty Dunn, Raja Kandaswamy, Erik B. Finger, David E.R. Sutherland, and Rajinder Singh for their kind assistance in these studies. We declare that the authors do not have any conflict of interests.
Disclosure of Potential Conflict of Interest
Dr. Ikramuddin serves on boards for Novo Nordisk, Medica, and OptumHealth, consults for Metamodix Inc. and Covidien, and has research grants from USGI Medical Inc., Enteromedics, Covidien, and ReShape Medical. Dr. Bernlohr is SAB member for Celladon Corp. For the remaining authors, no conflicts were declared.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Source of Funding
The Minnesota Obesity Center (NIH DK050456) and NIH DK084669.
Grant Support
American Diabetes Association (ADA 7-11-ST-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jahansouz, C., Serrot, F.J., Frohnert, B.I. et al. Roux-en-Y Gastric Bypass Acutely Decreases Protein Carbonylation and Increases Expression of Mitochondrial Biogenesis Genes in Subcutaneous Adipose Tissue. OBES SURG 25, 2376–2385 (2015). https://doi.org/10.1007/s11695-015-1708-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1708-5