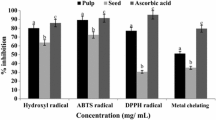
Abstract
This study was conducted to first determine the phenolic compounds and then the antioxidant activity of black plum fruit pulp and peel. For these characterisations, classic methods were used. Moreover, the ability of extracts to scavenge free radicals and their reducing power were measured according to 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) methods, respectively. The total polyphenol, flavonoid and anthocyanin contents of pulp and peel ranged from 202.51 ± 4.19 to 463.45 ± 6.85 mg gallic acid equivalent (GAE)/100 g of Dry Weight (DW), 75.71 ± 1.03 to 145.55 ± 1.03 mg quercetin equivalent (QE)/100 g DW, and from 1.91 ± 0.08 to 8.28 ± 0.83 mg cyanidin 3-O-β-D-glucoside equivalent (C3GE)/100 g DW respectively. However, these compounds were higher in peel extracts than in pulp extracts. In addition, peel extract showed the strongest antioxidant capacities. Significant correlations were found between methods applied to determine antioxidant activity (DPPH and FRAP) in black plum pulp and peel extracts and their total phenols and flavonoids contents. Cinnamic acid and gallic acid were mains phenols in pulp and peel extracts respectively, except fruits peel from Ferke where the main phenol was cinnamic acid. Thus, peel of black plum fruit could be used as an inexpensive and natural source of antioxidants and contribute to the prevention of degenerative diseases.



Similar content being viewed by others

References
H. Cai, D.G. Harrison, Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87, 840–844 (2000)
J. Himmelfarb, P. Stenvinkel, T.A. Ikizler, R.M. Hakim, Perspectives in renal medicine: the elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62(5), 1524–1538 (2002)
D. Harrison, K.K. Griendling, U. Landmesser, B. Hornig, H. Drexler, Role of oxidative stress in atherosclerosis. Am J Cardiol 91(3 SUPPL.), 7–11 (2003)
E. Takimoto et al., Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 115(5), 1221–1231 (2005)
G.E. Forcados, C.N. Chinyere, M.L. Shu, Acalypha wilkesiana: therapeutic and toxic potential. J Med Surg Pathol 1, 122 (2016)
M. Alpay, L.R.F. Backman, X.D. Cheng, M. Dukel, W.J. Kim, L.B. Ai, K.D. Brown, Oxidative stress shapes breast cancer phenotype through chronic activation of ATM-dependent signaling. Breast Cancer Res Treat 151, 75–87 (2015)
D.M. Small, J.S. Coombes, N. Bennett, D.W. Johnson, G.C. Gobe, Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 17(4), 311–321 (2012)
K. Apel, H. Hirt, Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55(1), 373–399 (2004)
M.P. Murphy, How mitochondria produce reactive oxygen species. Biochem J 417(1), 1–13 (2009)
S. Saha, L. Kumar, B.W. Soo, C. Jihye, Y.K. Hye, Y. Kyeongseok, M.D. Gwang, A.C. Ahmed, G. Ssang, Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci 18(7), 1544 (2017)
M. Sharifi-rad, N.V.A. Kumar, P. Zucca, E.M. Varoni, L. Dini, E. Panzarini, J. Rajkovic, P.F.T. Valere, E. Azzini, I. Peluso, A.P. Mishra, M. Nigam, A. Manisha, Lifestyle oxidative stress, and antioxidants : back and forth in the pathophysiology of chronic diseases. Front Physiol 11, 1–21 (2020)
A. Singh, R. Kukreti, L. Saso, Oxidative stress : a key modulator in neurodegenerative diseases. Molecules 24, 1583 (2019)
T. Tian, Z. Wang, J. Zhang, Review article pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev 2017, 4535194 (2017)
D. Haj Mouhamed, A. Ezzaher, F. Neffati, W. Douki, L. Gaha, M.F. Najjar, Étude d’un marqueur du stress oxydant chez les fumeurs: le malondialdéhyde. Immuno-Anal Biol Spec 27(4), 153–158 (2012)
N.D. Ouattara, E. Gaille, F.W. Stauffer, A. Bakayoko, Diversité floristique et ethnobotanique des plantes sauvages comestibles dans le Département de Bondoukou (Nord- Est de la Côte d ’ Ivoire ). J Appl Biol 98, 9284–9300 (2016)
F.K. Traore, P.A. Ahi, Y.K. Kone, D. Soro, E.N. Assidjo, Profils des consommateurs et caractérisations physiques et chimiques des fruits de la prune noire (Vitex doniana) du Département de Bondoukou au Nord-Est de la Côte d’Ivoire. Int J Innov Appl Stud 25(1), 121–130 (2018)
M. Tiébré, D. Ouattara, B. Tra, A. Vroh, A. Gnagbo, K. Edouard, Diversité floristique et disponibilité des plantes utilitaires en zone soudanienne de la Côte d ’ Ivoire. J Appl Biol 107, 9699–9707 (2016)
K.H. Soro, K.K. Youssouf, A.K. David, S. Doudjo, E.F. Eric, A.N. Emmanuel, Caractérisation Biochimique De La Pulpe des Fruits Du Prunier Noir (Vitex Doniana) De La Côte d’Ivoire. Eur Sci J 14(3), 252–270 (2018)
A.K. Martial-Didier, K.K. Hubert, K.E. Jean Parfait, T. Kablan, Phytochemical properties and proximate composition of papaya (Carica papaya L. var solo 8) peels. Turk J Agric 5(6), 676–680 (2017). https://doi.org/10.24925/turjaf.v5i6.676-680.1154
N.A. Abdul Aziz, L.M. Wong, R. Bhat, L.H. Cheng, Evaluation of processed green and ripe mango peel and pulp flours (Mangifera indica var. Chokanan) in terms of chemical composition, antioxidant compounds and functional properties. J Sci Food Agric 92(3), 557–563 (2012)
Z. Xue, W. Feng, J. Cao, D. Cao, W. Jiang, Antioxidant activity and total phenolic contents in peel and pulp of chinese jujube (ziziphus jujuba mill) fruits. J Food Biochem 33(5), 613–629 (2009). https://doi.org/10.1111/j.1745-4514.2009.00241.x
S. Gorinstein, O. Martin-belloso, A. Lojek, C. Milan, R. Soliva-fortuny, Y. Park, S. Trakhtenberg, Comparative content of some phytochemicals in Spanish apples, peaches and pears. J Sci Food Agric (2002). https://doi.org/10.1002/jsfa.1178
J.L. Mau, S.Y. Tsai, Y.H. Tseng, S.J. Huang, Antioxidant properties of methanolic extracts from Ganoderma tsugae. Food Chem 93(4), 641–649 (2005)
X. Gao, M. Ohlander, N. Jeppsson, L. Bjo, V. Trajkovski, Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem 48, 1485–1490 (2000)
A. Meda, C.E. Lamien, M. Romito, J. Millogo, O.G. Nacoulma, Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91(3), 571–577 (2005)
J. Lee, R.W. Durst, R.E. Wrolstad, Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J AOAC Int 88(5), 1269–1278 (2005)
W. Brand-Williams, M.E. Cuvelier, C. Berset, Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1), 25–30 (1995)
Y.C. Hseu et al., Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem Toxicol 46(1), 105–114 (2008)
H. Sakakibara, Y. Honda, S. Nakagawa, H. Ashida, K. Kanazawa, Simultaneous determination of all polyphenols in vegetables, fruits, and teas. Food Chem 51(3), 571–581 (2003)
M.C. Garau, S. Simal, C. Rosselló, A. Femenia, Effect of air-drying temperature 531 on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem 104(3), 1014–1024 (2007)
V. Goulas, G.A. Manganaris, Exploring the phytochemical content and the antioxidant potential of Citrus fruits grown in Cyprus. Food Chem 131(1), 39–47 (2012)
B. Levaj, D.U. Verica, D.B. Kovačević, N. Krasnići, Determination of flavonoids in pulp and peel of mandarin fruits. Agric Conspec Sci 74(3), 221–225 (2009)
M. Reza et al., Comparative antioxidant activity and total flavonoid content of Persian pomegranate (Punica granatum L.) cultivars. Iran J Pharm Res 10(3), 519–524 (2011)
D. Marinova, F. Ribarova, M. Atanassova, Total phenolics and total flavonoids in bulgarian fruits and vegetables. J Univ Chem Technol Met 40(3), 255 (2005)
D.L. Luthria, S. Mukhopadhyay, D.T. Krizek, Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J Food Compos Anal 19(8), 771–777 (2006)
L. Jaakola, A. Hohtola, Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ 33(8), 1239–1247 (2010)
U. Tiwari, E. Cummins, Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res Int 50(2), 497–506 (2013)
S.K. Lee, A.A. Kade, Pre-harvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol 20(3), 207–220 (2000)
U. Moor, K. Karp, P. Põldma, A. Pae, Cultural systems affect content of anthocyanins and vitamin C in strawberry fruits. Eur J Hortic Sci 70(4), 195–201 (2005)
C.M. Cantín, M.A. Moreno, Y. Gogorcena, Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) batsch] breeding progenies. J Agric Food Chem 57(11), 4586–4592 (2009)
S.M. Ribeiro, J.H. Queiroz, M.E. de Queiroz, F.M. Campos, Sant’Ana HM, “Antioxidant in mango (Mangifera indica L.) pulp.” Plant Foods Hum Nutr 62(1), 13–17 (2007)
P.M.B. Nihort, Chemical and organoleptic characterization of pawpaw and guava leathers. World J Agric Sci 1(1), 50–51 (2005)
M. Anttonen, R. Karjalainen, Environmental and genetic variation of phenolic compounds in red raspberry. J Food Compos Anal 18(8), 759–769 (2005)
Ndhala et al., Phenolic composition of Flacourtia indica, Opuntia megacantha and Sclerocarya birrea. Food Chem 103(1), 82–87 (2007)
J. Zhang et al., Effect and mechanism of action of cinnamic acid on the proliferation and apoptosis of Leukaemia cells. Biomed Res 25(3), 405–408 (2014)
F. Natella, M. Nardini, M. Di Felice, C. Scaccini, Benzoic and cinnamic acid derivatives as antioxidants: structure- activity relation. J Agric Food Chem 47(4), 1453–1459 (1999)
M. Strlič, T. Radovič, J. Kolar, B. Pihlar, Anti- and prooxidative properties of gallic acid in fenton-type systems. J Agric Food Chem 50(22), 6313–6317 (2002)
H. Gautier et al., How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J Agric Food Chem 56(4), 1241–1250 (2008)
E. Schwartz et al., Environmental conditions affect the color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J Agric Food Chem 57(19), 9197–9209 (2009)
F. Carbone et al., Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant Cell Environ 32(8), 1117–1131 (2009)
M.A. Rosales et al., The effect of environmental conditions on nutritional quality of cherry tomato fruits: evaluation of two experimental Mediterranean greenhouses. J Sci Food Agric 91(1), 152–162 (2011)
G.E. Pereira et al., Microclimate influence on mineral and metabolic profiles of grape berries. J Agric Food Chem 54(18), 6765–6775 (2006)
P.R. Jeyaramraja, P.K. Pius, R.R. Kumar, D. Jayakumar, Soil moisture stress-induced alterations in bioconstituents determining tea quality. J Sci Food Agric 83(12), 1187–1191 (2003)
M.H. Jang, X.L. Piao, J.M. Kim, S.W. Kwon, J.H. Park, Inhibition of cholinesterase and amyloid-&bgr; aggregation by resveratrol oligomers from Vitis amurensis. Phyther Res 22(4), 544–549 (2008)
J. Levett, Review article. Aust Libr J 35(1), 53–54 (1986)
G. Cao, R.L.P. Emin Sofic, Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 15, 749–760 (1988)
E.J. Lien, S. Ren, H.H. Bui, R. Wang, Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic Biol Med 26(3–4), 285–294 (1999)
E. Sariburun, S. Şahin, C. Demir, C. Türkben, V. Uylaşer, Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J Food Sci 75(4), 328–335 (2010)
K. Robards, P.D. Prenzler, G. Tucker, P. Swatsitang, W. Glover, Phenolic compounds and their role in oxidative processes in fruits. Food Chem 66(4), 401–436 (1999)
H. Palafox-carlos, E.M. Yahia, G.A. González-aguilar, Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo ) fruit by HPLC – DAD – MS / MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem 135(1), 105–111 (2012)
P. Reckziegel et al., Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats. Toxicol Rep 3, 351–356 (2016)
M. Goudarzi, M. Kalantar, H. Kalantar, The Hepatoprotective effect of gallic acid on mercuric chloride-induced liver damage in rats. Jundishapur J Nat Pharm Prod 13(3), e12450 (2017)
P.I. Schimites, H.J. Segat, L.G. Teixeira, L.R. Martins, L.T. Mangini, P.S. Baccin, A.V. Soares, Gallic acid prevents ketamine-induced oxidative damages in brain regions and liver of rats. Neurosci Lett 714, 134560 (2020)
J. Wu, H. Gao, L. Zhao, X. Liao, F. Chen, Z. Wang, X. Hu, Chemical compositional characterization of some apple cultivars. Food Chem 103, 88–93 (2007)
F. Vieira, G. Borges, C. Copetti, L. Gonzaga, E. Nunes, R. Fett, Activity and contents of polyphenolic antioxidants in the whole fruit, flesh and peel of three apple cultivars. Archivos Latinoamericanos de Nutrición 59, 101–106 (2009)
C. Henríquez, S. Almonacid, I. Chiffelle, T. Valenzuela, M. Araya, L. Cabezas, R. Simpson, H. Speisky, Determination of antioxidant capacity, total phenolic content and mineral composition of different fruit tissue of five apple cultivars grown in chile. Chil J Agric Res 70(4), 523–536 (2010)
C. Guo, J. Yang, J. Wei, Y. Li, J. Xu, Y. Jiang, Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res 23(12), 1719–1726 (2003)
J. Kolniak-Ostek, Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem 203, 491–497 (2016)
A. Lamien-Meda et al., Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 13(3), 581–594 (2008)
X. Ma et al., Polyphenolic compounds and antioxidant properties in mango fruits. Sci Hortic (Amsterdam) 129(1), 102–107 (2011)
T.A. Sokamte, P.D. Mbougueng, N.L. Tatsadjieu, N.M. Sachindra, Phenolic compounds characterization and antioxidant activities of selected spices from Cameroon. S Afr J Bot 121, 7–15 (2019)
L. Mira, M.T. Fernandez, M. Santos, R. Rocha, M.H. Florêncio, K.R. Jennings, Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res 36(11), 1199–1208 (2002)
C.L. Millar, Q. Duclos, C.N. Blesso, Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv Nutr 8(2), 226–239 (2017)
T.Y. Wang, Q. Li, K.S. Bi, Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci 13(1), 12–23 (2018)
S. Kiokias, C. Proestos, V. Oreopoulou, Effect of natural food antioxidants against ldl and dna oxidative changes. Antioxidants 7(10), 1–20 (2018)
Acknowledgements
We thank the Food Products Quality and Safety Laboratory of University of Liege – Gembloux AgroBioTech, especially the laboratory technicians and Dr Touré Yétioman.
Funding
There is no funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design and realization. Material preparation, data collection and analysis were performed by TKF and KKY. The first draft of the manuscript was written by TKF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Traore, K.F., Kone, K.Y., Ahi, A.P. et al. Phenolic compounds characterisation and antioxidant activity of black plum (Vitex doniana) fruit pulp and peel from Côte d’Ivoire. Food Measure 15, 1281–1293 (2021). https://doi.org/10.1007/s11694-020-00719-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00719-3