Abstract
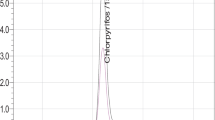
A simple analytical method was developed and validated in chilli, tomato, grape and mango fruits using liquid chromatography tandem mass spectrometry. The method comprised of extraction with ethyl acetate and cyclohexane mixture followed by d-SPE cleanup employing modified quick, easy, cheap, effective, rugged and safe extraction method and quantified in LC–MS/MS using gradient elution. The method was validated in concentration ranging from 0.01 to 0.1 µg g−1. The recovery of azoxystrobin in different crops was ranging from 84.36 to 95.64 % at three different concentration levels of analytes with relative standard deviation (HorRat < 20 %) of 4–14 %. The global uncertainty was calculated at limit of quantification level i.e. 0.01 µg g−1. In order to evaluate in safety use in India, a field study was conducted with the following extraction method. The calculated half life periods of azoxystrobin were ranging from 3.10–3.46, 3.64–3.46, 1.65–1.96 and 1.32–1.36 days respectively for experimental substrates. The PHI values of azoxystrobin in chilli, tomato, grape and mango fruits were determined as 4.76, 3.90, 4.06 and 10.74 days respectively.



Similar content being viewed by others
References
R.R. Karpate, R. Saxena, Post harvest profile of chilli. (Dept. of Ag. and Cooperation, Ministry of Agriculture, Nagpur, 2009), MRIN-2
FAO Statistical Database, FAOSTAT, (2007), http://www.fao.org. Accessed 29 June 2013
P.C. Adsule, Good Agricultural Practices for Production of Quality Table Grapes. (N.R.C for Grapes, Pune, 2013), pp. 38–57. www.drs.nio.org/drs/bitstream/2264/4073/1/Mar_Geol_307-310_88a.pdf. Accessed 18 Feb 2013
FAO database, Azoxystrobin: 229, (FAO/WHO Plant Production and protection 215, Ministry of Agriculture, Forestry and Fisheries, Tokyo, Japan, 2012), pp. 55–95. http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report12/JMPR_2012_Report.pdf. Accessed 18 June 2014
The gazette of India: Extraordinary (2009) Part II-Sec. 3(i): 8. (Dept. of Health and Public welfare, New Delhi), www.drugscontrol.org/GSR%20157%20(E)%20dtd%204.3.09.pdf. Accessed 30 July 2013
O. Golge, B. Kabak, J. Food Compos. Anal. 41, 86–97 (2015). doi:10.1016/j.jfca.2015.02.007
O. Golge, B. Kabak, Food Chem. 176, 319–332 (2015). doi:10.1016/j.jfca.2015.02.007
J.P. dos Anjos, J.B. de Andrade, Microchem. J. 120, 69–76 (2015). doi:10.1016/j.microc.2015.01.009
M.D.H. Prodhan, E.-N. Papadakis, E. Papadopoulou-Mourkidou, Int. J Environ. Anal. Chem. 1–11 (2015). doi:10.1080/03067319.2015.1025227
E. Szpyrka, A. Kurdziel, A. Matyaszek, M. Podbielska, J. Rupar, M. Słowik-Borowiec, Food Control 48, 137–142 (2015). doi:10.1016/j.foodcont.2014.05.039
B. Ivanova, M. Spiteller, Analyst 137(14), 3355–3364 (2012). doi:10.1039/C2AN35174A
M. Wilkinson, (1994) ICIA5504: Metabolism in winter wheat, Jealott’s Hill Research Station, Zeneca Agrochemicals, UK. FAO Report No. RJ1682B. Syngenta File No. ICI5504/0286
A. Boudina, C. Emmelin, A. Baaliouamer, O. Païssé, J.M. Chovelon, Chemosphere 68(7), 1280–1288 (2007). doi:10.1016/j.chemosphere.2007.01.051
K. Banerjee, A.P. Ligon, M. Spiteller, Anal. Bioanal. Chem. 382(7), 1527–1533 (2005). doi:10.1007/s00216-005-3336-8
K. Banerjee, A.P. Ligon, M. Spiteller, Agric. Food Chem. 54(25), 9479–9487 (2006). doi:10.1021/jf0620214
K. Banerjee, A.P. Ligon, M. Spiteller, Anal. Bioanal. Chem. 388(8), 1831–1838 (2007). doi:10.1007/s00216-007-1382-0
G. Anderson, M.E. Lewiston, Preparing solutions and making dilutions. (Mississippi Genome Exploration Laboratory, Mississippi State, MS) http://www.mgel.msstate.edu/pdf/solutions.pdf. Accessed 10 July 2014
A. Ben-David, C.E. Davidson, J. Microbiol. Methods 107, 214–221 (2014). doi:10.1016/j.mimet.2014.08.023
W. Horwitz, L.R. Kamps, K.W. Boyer, J. Assoc. Off. Anal. Chem. 63(6), 1344–1354 (1980)
Method validation and quality control procedures for pesticide residues analysis in food and feed (2009) Document No. SANCO/10684/2009. 6th EU AQC
European Union Commission Directorate of General Health and Consumer Protection (2000) Guidance document on residue analytical methods. Document No. SANCO/825/00 rev. 6
Quantifying uncertainty in analytical measurement, (2000) Document no. EURACHEM/CITAC Guide CG 4, 2nd ed., http://www.measurementuncertainty.org.html. Accessed 15 July 2013
W.M. Hoskins, FAO Plant Prot. Bull. 9, 163–168 (1961)
EFSA, Reasoned opinion on the review of the existing maximum residue levels (MRLs) for azoxystrobin according to Article 12 of Regulation (EC) No 396/20051, European Food Safety Authority (EFSA), Parma, Italy, EFSA Journal, 11(12): 3497–3594 (2013). http://www.efsa.europa.eu/en/efsajournal/doc/3497.pdf. Accessed 25 Sep 2014
Acknowledgments
Authors are thankful to Indofil Industries Limited, Mumbai for financial assistance and grateful to Department of Agril. Chemicals, Bidhan Chandra KrishiViswavidyalaya, Mohanpur, WB, India, for all instrumental facilities. They are also thankful to Department of Chemistry, Visva-Bharati, Santiniketan, WB, India for the general facilities to continue the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, S., Mukherjee, S., Das, G.K. et al. Analytical method validation and comparison of two extraction techniques for screening of azoxystrobin from widely used crops using LC–MS/MS. Food Measure 9, 517–524 (2015). https://doi.org/10.1007/s11694-015-9260-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-015-9260-5