Abstract
Background
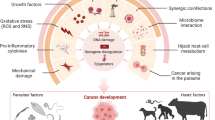
Multi-factorial reasons are an induction to cause cancer. Different infections and infestations with viruses, bacteria, and parasites have been detected for many years to be related to human carcinogenesis.
Purpose
The study aimed to review all ideas of tumor carcinogenesis and its associations with parasitic infections and infestations.
Methods
We reviewed several articles (published and imprinted) by selecting, extracting, and synthesizing data about the relationship between cancers and parasites.
Results
Several helminths infections as schistosomiasis, are highly carcinogenic agents for bladder cancer, whereas trypanosomiasis has a bi-model role in cancer development. Leishmaniasis may be a cause of hepatocarcinoma, skin cancer, and lymphomas. In addition, malaria appears to be causative in the carcinogenesis of some cancers; as Burkitt lymphoma. Also, data from previous studies suggested that Strongyloides stercoralis may be a relevant co-factor in lymphomas.
Conclusion
There are different mechanisms of parasitic infection to be enhancing in carcinogenesis of cancer in human.

Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
WHO (2015) Cancer Fact Sheet No. 297 (Feb). https://www.who.int/news-room/fact-sheets/detail/cancer
IARC (2012) Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog. Risks Hum. 100 (Pt B), 1–441. PMID: 23189750; PMCID: PMC4781184
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El GF et al (2009) A review of human carcinogens–part B: biological agents. Lancet Oncol 10(4):321–322. https://doi.org/10.1016/s1470-2045(09)70096-8
Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S (2012) Burkitt’s lymphoma. Lancet. 31;379(9822):1234-44. https://doi.org/10.1016/S0140-6736(11)61177-X
Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ (2007) Liver fluke induces cholangiocarcinoma. PLoS Med 4(7):e201. https://doi.org/10.1371/journal.pmed.0040201
Pakharukova MY, Mordvinov VA (2016) The liver fluke Opisthorchis felineus: biology, epidemiology and carcinogenic potential. Trans R Soc Trop Med Hyg 110(1):28–36. https://doi.org/10.1093/trstmh/trv085
Amin HAA, Kobaisi MH, Samir RM (2019) Schistosomiasis and Bladder Cancer in Egypt: Truths and Myths. Open Access Maced J Med Sci. 10;7(23):4023-9. https://doi.org/10.3889/oamjms.2019.857
Alsaad R, Hameed M (2021) The first record of zoonotic genes of cutaneous leishmaniasis among Human, Dogs, and sandflies by Nested polymerase chain reaction and phylogenetic analyses. Open Access Maced J Med Sci 29(A):610–621. https://doi.org/10.3889/oamjms.2021.6639
Alsaad RKA, Hameed MH (2021) An epidemiological study of cutaneous leishmaniosis in human and dogs. Ann Parasitol 67(3):417–433. https://doi.org/10.17420/ap6703.355
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6(7):411–425. https://doi.org/10.1016/S1473-3099(06)70521-7
Brindley PJ, da Costa JM, Sripa B (2015) Why does infection with some helminths cause cancer? Trends Cancer 1(13):174–182. https://doi.org/10.1016/j.trecan.2015.08.011
Knowles MA, Hurst CD (2015) Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 15(1):25–41. https://doi.org/10.1038/nrc3817
van Tong H, Brindley PJ, Meyer CG, Velavan TP (2017) Parasite infection, carcinogenesis and human malignancy. EBioMedicine 15:12–23. https://doi.org/10.1016/j.ebiom.2016.11.034
Honeycutt J, Hammam O, Hsieh MH (2015) Schistosoma haematobium egg-induced bladder urothelial abnormalities dependent on p53 are modulated by host sex. Exp Parasitol 158:55–60. https://doi.org/10.1016/j.exppara.2015.07.002
Qiu DC, Hubbard AE, Zhong B, Zhang Y, Spear RC (2005) A matched, case-control study of the association between Schistosoma japonicum and liver and colon cancers, in rural China. Ann Trop Med Parasitol 99(1):47–52. https://doi.org/10.1179/136485905X19883
Chen MG (2014) Assessment of morbidity due to Schistosoma japonicum infection in China. Infect Dis Poverty 3(1):6. https://doi.org/10.1186/2049-9957-3-6
Zanger P, Habscheid W, Kremsner PG, Dahm HH (2010) Schistosoma japonicum infection and rectal carcinoid tumour: underreported coincidence or neglected association? Epidemiol Infect 138(9):1289–1291. https://doi.org/10.1017/S095026880999152X
Basílio-de-Oliveira CA, Aquino A, Simon EF, Eyer-Silva WA (2002) Concomitant prostatic schistosomiasis and adenocarcinoma: case report and review. Braz J Infect Dis 6(1):45–49. https://doi.org/10.1590/s1413-86702002000100007
Salim H, Hamid OE, Mekki HK, Suleiman SO, Ibrahim SH SZ (2010) Colorectal carcinoma associated with schistosomiasis: a possible causal relationship. World J Surg Oncol 8:68. https://doi.org/10.1186/1477-7819-8-68
Kiremit MC, Cakir A, Arslan F, Ormeci T, Erkurt B, Albayrak S (2015) The bladder carcinoma secondary to schistosoma mansoni infection: a case report with review of the literature. Int J Surg Case Rep 13:76–78. https://doi.org/10.1016/j.ijscr.2015.05.038
Cheever AW, Lenzi JA, Lenzi HL, Andrade ZA (2002) Experimental models of Schistosoma mansoni infection. Mem Inst Oswaldo Cruz 97(7):917–940. https://doi.org/10.1590/s0074-02762002000700002
Zalata KR, Nasif WA, Ming SC, Lotfy M, Nada NA, El-Hak NG, Leech SH (2005) p53, Bcl-2 and C-Myc expressions in colorectal carcinoma associated with schistosomiasis in Egypt. Cell Oncol 27(4):245–253. https://doi.org/10.1155/2005/547010
Madbouly KM, Senagore AJ, Mukerjee A, Hussien AM, Shehata MA, Navine P, Delaney CP, Fazio VW (2007) Colorectal cancer in a population with endemic Schistosoma mansoni: is this an at-risk population? Int J Colorectal Dis 22(2):175–181. https://doi.org/10.1007/s00384-006-0144-3
Petney TN, Andrews RH, Saijuntha W, Wenz-Mücke A, Sithithaworn P (2013) The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol 43(12–13):1031–1046. https://doi.org/10.1016/j.ijpara.2013.07.007
Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B (2012) The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int 61(1):10–16. https://doi.org/10.1016/j.parint.2011.08.014
Forrer A, Sayasone S, Vounatsou P, Vonghachack Y, Bouakhasith D, Vogt S, Glaser R, Utzinger J, Akkhavong K, Odermatt P (2012) Spatial distribution of, and risk factors for, Opisthorchis viverrini infection in southern Lao PDR. PLoS Negl Trop Dis 6(2):e1481. https://doi.org/10.1371/journal.pntd.0001481
Xayaseng V, Phongluxa K, van Eeuwijk P, Akkhavong K, Odermatt P (2013) Raw fish consumption in liver fluke endemic areas in rural southern Laos. Acta Trop 127(2):105–111. https://doi.org/10.1016/j.actatropica.2013.03.016
Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A (2012) The tumorigenic liver fluke Opisthorchis viverrini–multiple pathways to cancer. Trends Parasitol 28(10):395–407. https://doi.org/10.1016/j.pt.2012.07.006
Keiser J, Utzinger J (2009) Food-borne trematodiases. Clin Microbiol Rev 22(3):466–483. https://doi.org/10.1128/CMR.00012-09
Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA (2007) Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer 120(3):638–641. https://doi.org/10.1002/ijc.22283
Palmer WC, Patel T (2012) Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 57(1):69–76. https://doi.org/10.1016/j.jhep.2012.02.022
Chaiyadet S, Smout M, Johnson M, Whitchurch C, Turnbull L, Kaewkes S, Sotillo J, Loukas A, Sripa B (2015) Excretory/secretory products of the carcinogenic liver fluke are endocytosed by human cholangiocytes and drive cell proliferation and IL6 production. Int J Parasitol 45(12):773–781. https://doi.org/10.1016/j.ijpara.2015.06.001
Chaiyadet S, Sotillo J, Smout M, Cantacessi C, Jones MK, Johnson MS, Turnbull L, Whitchurch CB, Potriquet J, Laohaviroj M, Mulvenna J, Brindley PJ, Bethony JM, Laha T, Sripa B, Loukas A (2015) Carcinogenic liver fluke secretes extracellular vesicles that promote cholangiocytes to adopt a tumorigenic phenotype. J Infect Dis 212(10):1636–1645. https://doi.org/10.1093/infdis/jiv291
Khuntikeo N, Loilome W, Thinkhamrop B, Chamadol N, Yongvanit P (2016) A Comprehensive Public Health Conceptual Framework and Strategy to effectively combat Cholangiocarcinoma in Thailand. PLoS Negl Trop Dis 10(1):e0004293. https://doi.org/10.1371/journal.pntd.0004293
Ninlawan K, O’Hara SP, Splinter PL, Yongvanit P, Kaewkes S, Surapaitoon A, LaRusso NF, Sripa B (2010) Opisthorchis viverrini excretory/secretory products induce toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol Int 59(4):616–621. https://doi.org/10.1016/j.parint.2010.09.008
Sripa B, Thinkhamrop B, Mairiang E, Laha T, Kaewkes S, Sithithaworn P, Periago MV, Bhudhisawasdi V, Yonglitthipagon P, Mulvenna J, Brindley PJ, Loukas A, Bethony JM (2012) Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS Negl Trop Dis 6(5):e1654. https://doi.org/10.1371/journal.pntd.0001654
Vale N, Gouveia MJ, Botelho M, Sripa B, Suttiprapa S, Rinaldi G, Gomes P, Brindley PJ, Correia da Costa JM (2013) Carcinogenic liver fluke Opisthorchis viverrini oxysterols detected by LC-MS/MS survey of soluble fraction parasite extract. Parasitol Int 62(6):535–542. https://doi.org/10.1016/j.parint.2013.08.001
Won J, Ju JW, Kim SM, Shin Y, Chung S, Pak JH (2014) Clonorchis sinensis infestation promotes three-dimensional aggregation and invasion of cholangiocarcinoma cells. PLoS One. 23;9(10):e110705. https://doi.org/10.1371/journal.pone.0110705
Lim MK, Ju YH, Franceschi S, Oh JK, Kong HJ, Hwang SS, Park SK, Cho SI, Sohn WM, Kim DI, Yoo KY, Hong ST, Shin HR (2006) Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg 75(1):93–96 PMID: 16837714
Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Jang KT, Lee NY, Kim S, Hong ST (2006) Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol 44(6):1066–1073. https://doi.org/10.1016/j.jhep.2005.11.040
Pak JH, Son WC, Seo SB, Hong SJ, Sohn WM, Na BK, Kim TS (2016) Peroxiredoxin 6 expression is inversely correlated with nuclear factor-κB activation during Clonorchis sinensis infestation. Free Radic Biol Med 99:273–285. https://doi.org/10.1016/j.freeradbiomed.2016.08.016
Maeng S, Lee HW, Bashir Q, Kim TI, Hong SJ, Lee TJ, Sohn WM, Na BK, Kim TS, Pak JH (2016) Oxidative stress-mediated mouse liver lesions caused by Clonorchis sinensis infection. Int J Parasitol 46(3):195–204. https://doi.org/10.1016/j.ijpara.2015.11.003
Maksimova GA, Zhukova NA, Kashina EV, Lvova MN, Katokhin AV, Tolstikova TG, Mordvinov VA (2015) Role of opisthorchis felineus on induction of bile duct cancer. Parazitologiia 49(1):3–11 Russian. PMID: 26016330
Saltykova IV, Ogorodova LM, Bragina EY, Puzyrev VP, Freidin MB (2014) Opisthorchis felineus liver fluke invasion is an environmental factor modifying genetic risk of atopic bronchial asthma. Acta Trop 139:53–56. https://doi.org/10.1016/j.actatropica.2014.07.004
Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW (2010) Estimating the global clinical burden of Plasmodium Falciparum malaria in 2007. PLoS Med 7(6):e1000290. https://doi.org/10.1371/journal.pmed.1000290
WHO, Factsheet on the World Malaria Report (2020) 2019. https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019
Wilmore JR, Asito AS, Wei C, Piriou E, Sumba PO, Sanz I, Rochford R (2015) AID expression in peripheral blood of children living in a malaria holoendemic region is associated with changes in B cell subsets and Epstein-Barr virus. Int J Cancer 136(6):1371–1380. https://doi.org/10.1002/ijc.29127
Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R (2007) Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria risk. Trop Med Int Health 12(8):936–943. https://doi.org/10.1111/j.1365-3156.2007.01875.x
Johnston WT, Mutalima N, Sun D, Emmanuel B, Bhatia K, Aka P, Wu X, Borgstein E, Liomba GN, Kamiza S, Mkandawire N, Batumba M, Carpenter LM, Jaffe H, Molyneux EM, Goedert JJ, Soppet D, Newton R, Mbulaiteye SM (2014) Relationship between Plasmodium Falciparum malaria prevalence, genetic diversity and endemic Burkitt lymphoma in Malawi. Sci Rep 17:4:3741. https://doi.org/10.1038/srep03741
Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, Kazura JW (2007) Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis 195(6):799–808. https://doi.org/10.1086/511984
Chattopadhyay PK, Chelimo K, Embury PB, Mulama DH, Sumba PO, Gostick E, Ladell K, Brodie TM, Vulule J, Roederer M, Moormann AM, Price DA (2013) Holoendemic malaria exposure is associated with altered Epstein-Barr virus-specific CD8(+) T-cell differentiation. J Virol 87(3):1779–1788. https://doi.org/10.1128/JVI.02158-12
Asito AS, Piriou E, Odada PS, Fiore N, Middeldorp JM, Long C, Dutta S, Lanar DE, Jura WG, Ouma C, Otieno JA, Moormann AM, Rochford R (2010) Elevated anti-zta IgG levels and EBV viral load are associated with site of tumor presentation in endemic Burkitt’s lymphoma patients: a case control study. Infect Agent Cancer 28:5:13. https://doi.org/10.1186/1750-9378-5-13
Tanaka T, Hirata T, Parrott G, Higashiarakawa M, Kinjo T, Kinjo T, Hokama A, Fujita J (2016) Relationship among Strongyloides stercoralis infection, human T-Cell lymphotropic virus type 1 infection, and Cancer: a 24-Year Cohort Inpatient Study in Okinawa, Japan. Am J Trop Med Hyg 94(2):365–370. https://doi.org/10.4269/ajtmh.15-0556
Satoh M, Toma H, Sugahara K, Etoh K, Shiroma Y, Kiyuna S, Takara M, Matsuoka M, Yamaguchi K, Nakada K, Fujita K, Kojima S, Hori E, Tanaka Y, Kamihira S, Sato Y, Watanabe T (2002) Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)25(+) HTLV-1-infected T-cells in human carriers of both HTLV-1 and S. Stercoralis. Oncogene 21(16):2466–2475. https://doi.org/10.1038/sj.onc.1205329
Seo AN, Goo YK, Chung DI, Hong Y, Kwon O, Bae HI (2015) Comorbid gastric adenocarcinoma and gastric and duodenal Strongyloides stercoralis infection: a case report. Korean J Parasitol 53(1):95–99. https://doi.org/10.3347/kjp.2015.53.1.95
Sava M, Huynh T, Frugoli A, Kong L, Salehpour M, Barrows B (2020) Colorectal Cancer related to Chronic Strongyloides stercoralis infection. Case Rep Gastrointest Med 2020(8886460). https://doi.org/10.1155/2020/8886460
Coura JR (2013) Chagas disease: control, elimination and eradication. Is it possible? Mem Inst Oswaldo Cruz 108(8):962–967. https://doi.org/10.1590/0074-0276130565
Stevens L, Dorn PL, Schmidt JO, Klotz JH, Lucero D, Klotz SA, Kissing (2011) bugs The vectors of Chagas. Adv Parasitol. 75:169 – 92. https://doi.org/10.1016/B978-0-12-385863-4.00008-3
Murta EF, Oliveira GP, Prado FO, De Souza MA, Tavares Murta BM, Adad SJ (2002) Association of uterine leiomyoma and Chagas’ disease. Am J Trop Med Hyg 66(3):321–324. https://doi.org/10.4269/ajtmh.2002.66.321
Adad SJ, Etchebehere RM, Araujo JR, Madureira AB, Lima VG, Silva AA, Eduardo C (2002) Association of chagasic megacolon and cancer of the colon: case report and review of the literature. Rev Soc Bras Med Trop 35(1):63–68. https://doi.org/10.1590/s0037-86822002000100012
Morsy TA (2013) Cutaneous leishmaniasis predisposing to human skin cancer: forty years local and regional studies. J Egypt Soc Parasitol 43(3):629–648. https://doi.org/10.12816/0006420
Carrillo-Larco RM, Acevedo-Rodriguez JG, Altez-Fernandez C, Ortiz-Acha K, Ugarte-Gil C (2019) Is there an association between cutaneous leishmaniasis and skin cancer? A systematic review. Wellcome Open Res 23:4:110. https://doi.org/10.12688/wellcomeopenres.15367.1
Kopterides P, Mourtzoukou EG, Skopelitis E, Tsavaris N, Falagas ME (2007) Aspects of the association between leishmaniasis and malignant disorders. Trans R Soc Trop Med Hyg 101(12):1181–1189. https://doi.org/10.1016/j.trstmh.2007.08.003
Putignani L, Menichella D (2010) Global distribution, public health and clinical impact of the protozoan pathogen Cryptosporidium. Interdiscip Perspect Infect Dis 2010(753512). https://doi.org/10.1155/2010/753512
Ryan U, Fayer R, Xiao L (2014) Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology 141:1667–1685. https://doi.org/10.1017/S0031182014001085
Bhalchandra S, Cardenas D, Ward HD (2018) Recent breakthroughs and ongoing limitations in Cryptosporidium research. F1000Research. 7:1380. https://doi.org/10.12688/f1000research.15333.1
Lilja M, Widerström M, Lindh J (2018) Persisting post-infection symptoms 2 years after a large waterborne outbreak of Cryptosporidium hominis in northern Sweden. BMC Res Notes 11:625. https://doi.org/10.1186/s13104-018-3721-y
Sawant M, Baydoun M, Creusy C, Chabé M, Viscogliosi E, Certad G, Benamrouz-Vanneste S (2020) Cryptosporidium and colon Cancer: cause or Consequence? Microorganisms 8(11):1665. https://doi.org/10.3390/microorganisms8111665
de Souza Ldo R, Rodrigues MA, Morceli J, Kemp R, Mendes RP (2004) Cryptosporidiosis of the biliary tract mimicking pancreatic cancer in an AIDS patient. Rev Soc Bras Med Trop 37(2):182–185. https://doi.org/10.1590/s0037-86822004000200015
Shebl FM, Engels EA, Goedert JJ (2012) Opportunistic intestinal infections and risk of colorectal cancer among people with AIDS. AIDS Res Hum Retroviruses 28(9):994–999. https://doi.org/10.1089/AID.2011.0185
Leven EA, Maffucci P, Ochs HD, Scholl PR, Buckley RH, Fuleihan RL, Geha RS, Cunningham CK, Bonilla FA, Conley ME, Ferdman RM, Hernandez-Trujillo V, Puck JM, Sullivan K, Secord EA, Ramesh M, Cunningham-Rundles C (2016) Hyper IgM Syndrome: a report from the USIDNET Registry. J Clin Immunol 36(5):490–501. https://doi.org/10.1007/s10875-016-0291-4
Osman M, Benamrouz S, Guyot K, Baydoun M, Frealle E, Chabe M, Gantois N, Delaire B, Goffard A, Aoun A, Jurdi N, Dabboussi F, Even G, Slomianny C, Gosset P, Hamze M, Creusy C, Viscogliosi E, Certad G (2017) High association of Cryptosporidium spp. infection with colon adenocarcinoma in Lebanese patients. PLoS ONE 12(12):e0189422. https://doi.org/10.1371/journal.pone.0189422
Baydoun M, Vanneste SB, Creusy C, Guyot K, Gantois N, Chabe M, Delaire B, Mouray A, Baydoun A, Forzy G, Chieux V, Gosset P, Senez V, Viscogliosi E, Follet J, Certad G (2017) Three-dimensional (3D) culture of adult murine colon as an in vitro model of cryptosporidiosis: Proof of concept. Sci Rep 7(1):17288. https://doi.org/10.1038/s41598-017-17304-2
Sulzyc-Bielicka V, Kuźna-Grygiel W, Kołodziejczyk L, Bielicki D, Kładny J, Stepień-Korzonek M, Telatyńska-Smieszek B (2007) Cryptosporidiosis in patients with colorectal cancer. J Parasitol 93(3):722–724. https://doi.org/10.1645/GE-1025R1.1
Sulżyc-Bielicka V, Kołodziejczyk L, Jaczewska S, Bielicki D, Kładny J, Safranow K (2012) Prevalence of Cryptosporidium sp. in patients with colorectal cancer. Pol Przegl Chir 84(7):348–351. https://doi.org/10.2478/v10035-012-0058-4
Sulżyc-Bielicka V, Kołodziejczyk L, Jaczewska S, Bielicki D, Safranow K, Bielicki P, Kładny J, Rogowski W (2018) Colorectal cancer and Cryptosporidium spp. infection. PLoS ONE 13(4):e0195834. https://doi.org/10.1371/journal.pone.0195834
Kopacz Ż, Kváč M, Karpiński P, Hendrich AB, Sąsiadek MM, Leszczyński P, Sak B, McEvoy J, Kicia M (2019) The First evidence of Cryptosporidium meleagridis infection in a Colon Adenocarcinoma from an Immunocompetent patient. Front Cell Infect Microbiol 9:35. https://doi.org/10.3389/fcimb.2019.00035
Zhang N, Yu X, Zhang H, Cui L, Li X, Zhang X, Gong P, Li J, Li Z, Wang X, Li X, Li T, Liu X, Yu Y, Zhang X (2020) Prevalence and genotyping of Cryptosporidium parvum in Gastrointestinal Cancer patients. J Cancer 11(11):3334–3339. https://doi.org/10.7150/jca.42393
Abd El-Latif NF, Kandil NS, Shamseya M, Elwany YN, Ibrahim HS (2023) Role of Cryptosporidium spp in Development of Colorectal Cancer. Asian Pac J Cancer Prev 24(2):667–674. https://doi.org/10.31557/APJCP.2023.24.2.667
Funding
Self-funded this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This study was approved by Baghdad medical city complex Committee Board (ID No. 101/ 2021).
Conflict of Interest
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alshewered, A.S. The Parasitism and Tumors Carcinogenesis: A Review Subject. Acta Parasit. 69, 183–189 (2024). https://doi.org/10.1007/s11686-024-00832-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-024-00832-z