Abstract
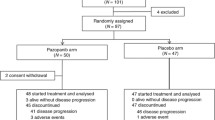
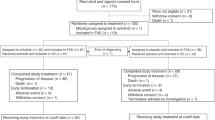
Patients with small-cell lung cancer (SCLC) relapse within months after completing previous therapies. This study aimed to investigate the efficacy and safety of anlotinib as third- or further-line therapy in patients with short-term relapsed SCLC from ALTER1202. Patients with short-term relapsed SCLC (disease progression within 3 months after completing ⩾ two lines of chemotherapy) in the anlotinib (n = 67) and placebo (n = 34) groups were analyzed. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival, objective response rate (ORR), disease control rate, and safety. Anlotinib significantly improved median PFS/OS (4.0 vs. 0.7 months, P < 0.0001)/(7.3 vs. 4.4 months, P = 0.006) compared with placebo. The ORR was 4.5%/2.9% in the anlotinib/placebo group (P = 1.000). The DCR in the anlotinib group was higher than that in the placebo group (73.1% vs. 11.8%, P < 0.001). The most common adverse events (AEs) were hypertension (38.8%), loss of appetite (28.4%), and fatigue (22.4%) in the anlotinib group and gammaglutamyl transpeptidase elevation (20.6%) in the placebo group. No grade 5 AEs occurred. For patients with short-term relapsed SCLC, third- or further-line anlotinib treatment was associated with improved survival benefit. Further studies are warranted in this regard.
Similar content being viewed by others
References
Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol 2019; 12(1): 47
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70(1): 7–30
Rudin CM, Giaccone G, Ismaila N. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Oncol Pract 2016; 12(1): 83–86
Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E; ESMO Guidelines Working Group. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl 6): vi99–vi105
Zimmerman S, Das A, Wang S, Julian R, Gandhi L, Wolf J. 2017–2018 scientific advances in thoracic oncology: small cell lung cancer. J Thorac Oncol 2019; 14(5): 768–783
Ito T, Kudoh S, Ichimura T, Fujino K, Hassan WA, Udaka N. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum Cell 2017; 30(1): 1–10
Waqar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC). Pharmacol Ther 2017; 180: 16–23
MacCallum C, Gillenwater HH. Second-line treatment of small-cell lung cancer. Curr Oncol Rep 2006; 8(4): 258–264
von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A, Carmichael J, Krebs JB, Ross G, Lane SR, Gralla R. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999; 17(2): 658–667
Ardizzoni A, Tiseo M, Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer 2014; 50(13): 2211–2218
Asai N, Ohkuni Y, Kaneko N, Yamaguchi E, Kubo A. Relapsed small cell lung cancer: treatment options and latest developments. Ther Adv Med Oncol 2014; 6(2): 69–82
Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, Spigel DR, Moreno V, Chau I, Hann CL, Eder JP, Steele NL, Pieters A, Fairchild J, Antonia SJ. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol 2019; 14(2): 237–244
Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH Jr, Delord JP, Gao B, Planchard D, Gottfried M, Zer A, Jalal SI, Penel N, Mehnert JM, Matos I, Bennouna J, Kim DW, Xu L, Krishnan S, Norwood K, Ott PA. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol 2020; 15(4): 618–627
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, Lou L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 2018; 109(4): 1207–1219
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018; 11(1): 120
Chen D, Xu J, Zhao Y, Chu T, Zhong H, Han B, Zhong R. Prognostic value of tumor cavitation in extensive-stage small-cell lung cancer patients treated with anlotinib. J Cancer Res Clin Oncol 2020; 146(2): 401–406
Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, Cheng Y, He J, Shi Y, Zhao Y, Yu H, Zhao Y, Chen W, Luo Y, Wu L, Wang X, Pirker R, Nan K, Jin F, Dong J, Li B, Sun Y. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol 2018; 4(11): 1569–1575
Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, Han B, Chen G, He J, Wang J, Lou D, Yu H, Wang S, Qin H, Li X. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled phase 2 study. Br J Cancer 2021; 125(3): 366–371
Cheng Y, Wang Q, Li K, Shi J, Wu L, Han B, Chen G, He J, Wang J, Qin H, Li X. OA13.03 Anlotinib as third-line or further-line treatment in relapsed SCLC: a multicentre, randomized, double-blind phase 2 trial. J Thorac Oncol 2018; 13(10): S351–S352
Kim YH, Mishima M. Second-line chemotherapy for small-cell lung cancer (SCLC). Cancer Treat Rev 2011; 37(2): 143–150
Simos D, Sajjady G, Sergi M, Liew MS, Califano R, Ho C, Leighl N, White S, Summers Y, Petrcich W, Wheatley-Price P. Third-line chemotherapy in small-cell lung cancer: an international analysis. Clin Lung Cancer 2014; 15(2): 110–118
Pietanza MC, Kadota K, Huberman K, Sima CS, Fiore JJ, Sumner DK, Travis WD, Heguy A, Ginsberg MS, Holodny AI, Chan TA, Rizvi NA, Azzoli CG, Riely GJ, Kris MG, Krug LM. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 2012; 18(4): 1138–1145
Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, Glisson BS, Farago AF, Dowlati A, Rudin CM, Le Moulec S, Lally S, Yalamanchili S, Wolf J, Govindan R, Carbone DP. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res 2019; 25(23): 6958–6966
Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, Antonia SJ, Ascierto PA, Moreno V, Atmaca A, Salvagni S, Taylor M, Amin A, Camidge DR, Horn L, Calvo E, Li A, Lin WH, Callahan MK, Spigel DR. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol 2020; 15(3): 426–435
Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou J, Lu Y, Shi Y, Wang Z, Jiang L, Luo Y, Zhang Y, Huang C, Li Q, Wu G. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018; 118(5): 654–661
Wu D, Nie J, Hu W, Dai L, Zhang J, Chen X, Ma X, Tian G, Han J, Han S, Long J, Wang Y, Zhang Z, Fang J. A phase II study of anlotinib in 45 patients with relapsed small cell lung cancer. Int J Cancer 2020; 147(12): 3453–3460
Acknowledgements
The authors wish to thank all the participating patients and their families, study personnel at all sites, and the ALTER 1202 clinical trial team. This study was supported by the Jilin Provincial Health and Family Planning Commission (No. 2019J077), the Science and Technology Agency of Jilin Provincial Project (No. 20200201518JC), and Chia-tai Tianqing Pharmaceutical Group Co., Ltd. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Jianhua Shi, Ying Cheng, Qiming Wang, Kai Li, Lin Wu, Baohui Han, Gongyan Chen, Jianxing He, Jie Wang, Haifeng Qin, and Xiaoling Li declare that they have no conflict of interest. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Shi, J., Cheng, Y., Wang, Q. et al. Anlotinib as third- or further-line therapy for short-term relapsed small-cell lung cancer: subgroup analysis of a randomized phase 2 study (ALTER1202). Front. Med. 16, 766–772 (2022). https://doi.org/10.1007/s11684-021-0916-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-021-0916-8