Abstract
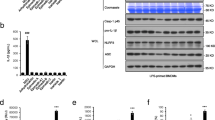
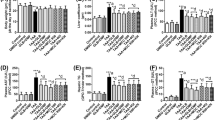
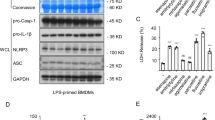
Psoraleae Fructus (PF) is a well-known traditional herbal medicine in China, and it is widely used for osteoporosis, vitiligo, and other diseases in clinical settings. However, liver injury caused by PF and its preparations has been frequently reported in recent years. Our previous studies have demonstrated that PF could cause idiosyncratic drug-induced liver injury (IDILI), but the mechanism underlying its hepatotoxicity remains unclear. This paper reports that bavachin isolated from PF enhances the specific stimuli-induced activation of the NLRP3 inflammasome and leads to hepatotoxicity. Bavachin boosts the secretion of IL-1β and caspase-1 caused by ATP or nigericin but not those induced by poly(I:C), monosodium urate crystal, or intracellular lipopolysaccharide. Bavachin does not affect AIM2 or NLRC4 inflammasome activation. Mechanistically, bavachin specifically increases the production of nigericin-induced mitochondrial reactive oxygen species among the most important upstream events in the activation of the NLRP3 inflammasome. Bavachin increases the levels of aspartate transaminase and alanine aminotransferase in serum and hepatocyte injury accompanied by the secretion of IL-1β via a mouse model of lipopolysaccharide-mediated susceptibility to IDILI. These results suggest that bavachin specifically enhances the ATP- or nigericin-induced activation of the NLRP3 inflammasome. Bavachin also potentially contributes to PF-induced idiosyncratic hepatotoxicity. Moreover, bavachin and PF should be evaded among patients with diseases linked to the ATP- or nigericin-mediated activation of the NLRP3 inflammasome, which may be a dangerous factor for liver injury.
Similar content being viewed by others
References
Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology 2014; 146(4): 914–928
Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf 2007; 6(6): 673–684
Deng X, Stachlewitz RF, Liguori MJ, Blomme EA, Waring JF, Luyendyk JP, Maddox JF, Ganey PE, Roth RA. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J Pharmacol Exp Ther 2006; 319(3): 1191–1199
Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther 2003; 307(1): 9–16
Dugan CM, MacDonald AE, Roth RA, Ganey PE. A mouse model of severe halothane hepatitis based on human risk factors. J Pharmacol Exp Ther 2010; 333(2): 364–372
Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. Lipopolysac-charide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci 2007; 100(1): 259–266
Metushi IG, Hayes MA, Uetrecht J. Treatment of PD-1−/− mice with amodiaquine and anti-CTLA4 leads to liver injury similar to idiosyncratic liver injury in patients. Hepatology 2015; 61(4): 1332–1342
Mak A, Uetrecht J. The role of CD8 T cells in amodiaquine-induced liver injury in PD1−/− mice cotreated with anti-CTLA-4. Chem Res Toxicol 2015; 28(8): 1567–1573
Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell 2016; 165(4): 792–800
He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 2016; 41(12): 1012–1021
Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014; 157(5): 1013–1022
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009; 458(7237): 514–518
Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, Vance RE. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 2017; 46(4): 649–659
Liu J, Berthier CC, Kahlenberg JM. Enhanced inflammasome activity in systemic lupus erythematosus is mediated via type I interferon-induced up-regulation of interferon regulatory factor 1. Arthritis Rheumatol 2017; 69(9): 1840–1849
Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti TD, van Loo G, Lamkanfi M. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 2014; 512(7512): 69–73
Goldberg EL, Asher JL, Molony RD, Shaw AC, Zeiss CJ, Wang C, Morozova-Roche LA, Herzog RI, Iwasaki A, Dixit VD. β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep 2017; 18(9): 2077–2087
Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases—did Warburg miss inflammation? Nat Immunol 2012; 13(4): 352–357
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013; 493(7434): 674–678
Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci 2014; 1319(1): 82–95
Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol 2012; 57(3): 642–654
Kato R, Uetrecht J. Supernatant from hepatocyte cultures with drugs that cause idiosyncratic liver injury activates macrophage inflammasomes. Chem Res Toxicol 2017; 30(6): 1327–1332
Wang Z, Xu G, Zhan X, Liu Y, Gao Y, Chen N, Guo Y, Li R, He T, Song X, Niu M, Wang J, Bai Z, Xiao X. Carbamazepine promotes specific stimuli-induced NLRP3 inflammasome activation and causes idiosyncratic liver injury in mice. Arch Toxicol 2019; 93(12): 3585–3599
Calitz C, du Plessis L, Gouws C, Steyn D, Steenekamp J, Muller C, Hamman S. Herbal hepatotoxicity: current status, examples, and challenges. Expert Opin Drug Metab Toxicol 2015; 11(10): 1551–1565
He J, Li X, Wang Z, Bennett S, Chen K, Xiao Z, Zhan J, Chen S, Hou Y, Chen J, Wang S, Xu J, Lin D. Therapeutic anabolic and anticatabolic benefits of natural Chinese medicines for the treatment of osteoporosis. Front Pharmacol 2019; 10: 1344
Gianfaldoni S, Wollina U, Tirant M, Tchernev G, Lotti J, Satolli F, Rovesti M, França K, Lotti T. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci 2018; 6(1): 203–207
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. 2015 edition. Part 1. Beijing: China Medical Science Press, 2015 (in Chinese)
Nam SW, Baek JT, Lee DS, Kang SB, Ahn BM, Chung KW. A case of acute cholestatic hepatitis associated with the seeds of Psoralea corylifolia (Boh-Gol-Zhee). Clin Toxicol (Phila) 2005; 43(6): 589–591
Gao Y, Wang Z, Tang J, Liu X, Shi W, Qin N, Wang X, Pang Y, Li R, Zhang Y, Wang J, Niu M, Bai Z, Xiao X. New incompatible pair of TCM: Epimedii Folium combined with Psoraleae Fructus induces idiosyncratic hepatotoxicity under immunological stress conditions. Front Med 2020; 14(1): 68–80
Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, Han QY, Wang H, Chen Y, Li HY, Li AL, Zhang XM, Zhou T, Li T. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell 2017; 68(1): 185–197. e6
He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, Liu Q, Liang G, Deng X, Jiang W, Zhou R. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun 2018; 9(1): 2550
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006; 440(7081): 228–232
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440(7081): 237–241
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature 2011; 479(7371): 117–121
Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013; 341(6151): 1246–1249
Dick MS, Sborgi L, Rühl S, Hiller S, Broz P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun 2016; 7(1): 11929
Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol 2010; 40(3): 620–623
Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013; 38(6): 1142–1153
Roth RA, Ganey PE. Animal models of idiosyncratic drug-induced liver injury—current status. Crit Rev Toxicol 2011; 41(9): 723–739
He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 2016; 41(12): 1012–1021
Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015; 21(3): 248–255
China Food And Drug Administration. Alert to liver damage caused by Zhuangguguanjiewan pills. Chin Community Doctors (Zhongguo She Qu Yi Shi) 2009; 25(374): 21 (in Chinese)
Li W, Peng DB. Analysis of adverse reactions cases of Xianlinggubao capsule. Chin J Pharmacovigilance (Zhongguo Yao Wu Jing Jie) 2011; 8(9): 555–556 (in Chinese)
Teschke R, Bahre R. Severe hepatotoxicity by Indian Ayurvedic herbal products: a structured causality assessment. Ann Hepatol 2009; 8(3): 258–266
Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, Devoogdt N, Lambrecht BN, Beschin A, Guilliams M. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun 2016; 7(1): 10321
Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 2017; 17(5): 306–321
Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol 2017; 66(6): 1300–1312
Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol 2016; 13(3): 316–327
Li J, Zhao J, Xu M, Li M, Wang B, Qu X, Yu C, Hang H, Xia Q, Wu H, Sun X, Gu J, Kong X. Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia-reperfusion injury. Cell Death Dis 2020; 11(4): 244
Ko M S, Yun J Y, Baek I J, Jang J E, Hwang J J, Lee S E, Heo S H, Bader D A, Lee C H, Han J, Moon J S, Lee J M, Hong E G, Lee I K, Kim S W, Park J Y, Hartig S M, Kang U J, Moore D D, Koh E H, Lee K U. Mitophagy deficiency increases NLRP3 to induce brown fat dysfunction in mice. Autophagy 2020; [Epub ahead of print] doi: https://doi.org/10.1080/15548627.2020.1753002
Yang G, Jang JH, Kim SW, Han SH, Ma KH, Jang JK, Kang HC, Cho YY, Lee HS, Lee JY. Sweroside prevents non-alcoholic steatohepatitis by suppressing activation of the NLRP3 inflammasome. Int J Mol Sci 2020; 21(8): 2790
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21(7): 677–687
Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 2015; 12(7): 387–400
Mak A, Uetrecht J. The combination of anti-CTLA-4 and PD1−/− mice unmasks the potential of isoniazid and nevirapine to cause liver injury. Chem Res Toxicol 2015; 28(12): 2287–2291
Boaru SG, Borkham-Kamphorst E, Van de Leur E, Lehnen E, Liedtke C, Weiskirchen R. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem Biophys Res Commun 2015; 458(3): 700–706
Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 2013; 14(5): 454–460
O’Neill LA. Cardiolipin and the Nlrp3 inflammasome. Cell Metab 2013; 18(5): 610–612
Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013; 39(2): 311–323
Goldman SJ, Taylor R, Zhang Y, Jin S. Autophagy and the degradation of mitochondria. Mitochondrion 2010; 10(4): 309–315
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011; 469(7329): 221–225
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012; 36(3): 401–414
Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 2011; 187(2): 613–617
Yang Y, Tang X, Hao F, Ma Z, Wang Y, Wang L, Gao Y. Bavachin induces apoptosis through mitochondrial regulated ER stress pathway in HepG2 cells. Biol Pharm Bull 2018; 41(2): 198–207
Yuan Z, Hasnat M, Liang P, Yuan Z, Zhang H, Sun L, Zhang L, Jiang Z. The role of inflammasome activation in triptolide-induced acute liver toxicity. Int Immunopharmacol 2019; 75: 105754
Sun W, Zeng C, Liu S, Fu J, Hu L, Shi Z, Yue D, Ren Z, Zhong Z, Zuo Z, Cao S, Peng G, Deng J, Hu Y. Ageratina adenophora induces mice hepatotoxicity via ROS-NLRP3-mediated pyroptosis. Sci Rep 2018; 8(1): 16032
Acknowldgements
This work has been supported by the Beijing Nova Program (No. Z181100006218001), the National Natural Science Foundation of China (Nos. 81874368, 81630100, 81903891, and 81573676), the National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” (Nos. 2017ZX09301022 and 2018ZX09101002-001-002), and the Innovation Groups of the National Natural Science Foundation of China (No. 81721002).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Compliance with ethics guidelines
Nan Qin, Guang Xu, Yan Wang, Xiaoyan Zhan, Yuan Gao, Zhilei Wang, Shubin Fu, Wei Shi, Xiaorong Hou, Chunyu Wang, Ruisheng Li, Yan Liu, Jiabo Wang, Haiping Zhao, Xiaohe Xiao, and Zhaofang Bai declare that they have no conflict of interest. This study was ratified by the Experimental Animal Center of the Fifth Medical Centre, Chinese PLA General Hospital in Beijing, China. Approval for the animal experimental research was in accordance with the ethical standards of the Ethics Committee in the Fifth Medical Centre, Chinese PLA General Hospital.
Rights and permissions
About this article
Cite this article
Qin, N., Xu, G., Wang, Y. et al. Bavachin enhances NLRP3 inflammasome activation induced by ATP or nigericin and causes idiosyncratic hepatotoxicity. Front. Med. 15, 594–607 (2021). https://doi.org/10.1007/s11684-020-0809-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-020-0809-2