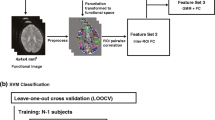
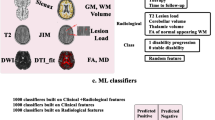
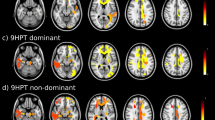
Abstract
Motor disability is a dominant and restricting symptom in multiple sclerosis, yet its neuroimaging correlates are not fully understood. We apply statistical and machine learning techniques on multimodal neuroimaging data to discriminate between multiple sclerosis patients and healthy controls and to predict motor disability scores in the patients. We examine the data of sixty-four multiple sclerosis patients and sixty-five controls, who underwent the MRI examination and the evaluation of motor disability scales. The modalities used comprised regional fractional anisotropy, regional grey matter volumes, and functional connectivity. For analysis, we employ two approaches: high-dimensional support vector machines run on features selected by Fisher Score (aiming for maximal classification accuracy), and low-dimensional logistic regression on the principal components of data (aiming for increased interpretability). We apply analogous regression methods to predict symptom severity. While fractional anisotropy provides the classification accuracy of 96.1% and 89.9% with both approaches respectively, including other modalities did not bring further improvement. Concerning the prediction of motor impairment, the low-dimensional approach performed more reliably. The first grey matter volume component was significantly correlated (R = 0.28-0.46, p < 0.05) with most clinical scales. In summary, we identified the relationship between both white and grey matter changes and motor impairment in multiple sclerosis. Furthermore, we were able to achieve the highest classification accuracy based on quantitative MRI measures of tissue integrity between patients and controls yet reported, while also providing a low-dimensional classification approach with comparable results, paving the way to interpretable machine learning models of brain changes in multiple sclerosis.




Similar content being viewed by others
Data availability
The raw imaging data were measured in the Institute for Clinical and Experimental Medicine and are not publicly available due to restrictions imposed by the administering institution. Raw anonymized data can be shared based on a formal data sharing agreement based on a submission of a project outline, that may - depending on the nature of the data requested - require project amendment approval from the local ethics committee. Informal requests for information shall be sent to the corresponding author. Part of the preprocessed fMRI data is available for download (Bučková et al. 2021).
References
Asch, P. V. (2011). Impact of mobility impairment in multiple sclerosis 2 - patients’ perspectives. European Neurological Review, 6(2), 115–120.
Basser, P. J., Mattiello, J., & LeBihan, D. (1994). MR diffusion tensor spectroscopy and imaging. Biophysical Journal, 66(1), 259–267.
Bendfeldt, K., Klöppel, S., Nichols, T. E., Smieskova, R., Kuster, P., Traud, S., Mueller-Lenke, N., Naegelin, Y., Kappos, L., Radue, E.-W., & Borgwardt, S. J. (2012). Multivariate pattern classification of gray matter pathology in multiple sclerosis. NeuroImage, 60(1), 400–408.
Berg, K., Wood-Dauphine, S., Williams, J. I., & Gayton, D. (2009). Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada, 41(6), 304–311.
Bozzali, M., Cercignani, M., Sormani, M. P., Comi, G., & Filippi, M. (2002). Quantification of brain gray matter damage in different MS phenotypes by use of diffusion tensor MR imaging. American Journal of Neuroradiology, 23(6), 985–988.
Bučková, B., Kopal, J., Řasová, K., Tintěra, J., & Hlinka, J. (2021). Open access: The effect of neurorehabilitation on multiple sclerosis–unlocking the resting-state fMRI data. Frontiers in Neuroscience, 15, 615.
Burschka, J. M., Keune, P. M., Menge, U., Oy, U.H.-V., Oschmann, P., & Hoos, O. (2012). An exploration of impaired walking dynamics and fatigue in multiple sclerosis. BMC Neurology, 12(1), 161.
Charalambous, T., Tur, C., Prados, F., Kanber, B., Chard, D. T., Ourselin, S., Clayden, J. D., Wheeler-Kingshott, C. A. M. G., Thompson, A. J., & Toosy, A. T. (2019). Structural network disruption markers explain disability in multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry, 90(2), 219–226.
Dobson, R., & Giovannoni, G. (2019). Multiple sclerosis-a review. European Journal of Neurology, 26(1), 27–40.
Filippi, M., Brück, W., Chard, D., Fazekas, F., Geurts, J. J. G., Enzinger, C., Hametner, S., Kuhlmann, T., Preziosa, P., Rovira, A., Schmierer, K., Stadelmann, C., & Rocca, M. A. (2019). Association between pathological and MRI findings in multiple sclerosis. The Lancet Neurology, 18(2), 198–210.
Filippi, M., & Rocca, M. A. (2011). MR imaging of multiple sclerosis. Radiology, 259(3), 659–681.
Fischer, J. S., Rudick, R. A., Cutter, G. R., & Reingold, S. C. (1999). The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Multiple Sclerosis Journal, 5(4), 244–250.
Giannì, C., Prosperini, L., Jonsdottir, J., & Cattaneo, D. (2014). A systematic review of factors associated with accidental falls in people with multiple sclerosis: a meta-analytic approach. Clinical Rehabilitation, 28(7), 704–716.
Heesen, C., Böhm, J., Reich, C., Kasper, J., Goebel, M., & Gold, S. (2008). Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Multiple Sclerosis Journal, 14(7), 988–991.
Hlinka, J., Paluš, M., Vejmelka, M., Mantini, D., & Corbetta, M. (2011). Functional connectivity in resting-state fMRI: Is linear correlation sufficient? NeuroImage, 54(3), 2218–2225.
Hobart, J., Lamping, D., Fitzpatrick, R., Riazi, A., & Thompson, A. (2001). The multiple sclerosis impact scale (MSIS-29)a new patient-based outcome measure. Brain, 124(5), 962–973.
Hobart, J., Riazi, A., Lamping, D., Fitzpatrick, R., & Thompson, A. (2003). Measuring the impact of MS on walking ability: The 12-item MS walking scale (MSWS-12). Neurology, 60(1), 31–36.
Jakimovski, D., Weinstock-Guttman, B., Hagemeier, J., Vaughn, C. B., Kavak, K. S., Gandhi, S., Bennett, S. E., Fuchs, T. A., Bergsland, N., Dwyer, M. G., Benedict, R. H. B., & Zivadinov, R. (2018). Walking disability measures in multiple sclerosis patients: Correlations with MRI-derived global and microstructural damage. Journal of the Neurological Sciences, 393, 128–134.
Keller, S. S., & Roberts, N. (2008). Voxel-based morphometry of temporal lobe epilepsy: An introduction and review of the literature. Epilepsia, 49(5), 741–757.
Kellner, E., Dhital, B., Kiselev, V. G., & Reisert, M. (2016). Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine, 76(5), 1574–1581.
LaRocca, N. G. (2011). Impact of walking impairment in multiple sclerosis. The Patient: Patient-Centered Outcomes Research, 4(3), 189–201.
Leray, E., Moreau, T., Fromont, A., & Edan, G. (2016). Epidemiology of multiple sclerosis. Revue Neurologique, 172(1), 3–13.
Martin, C. L., Phillips, B. A., Kilpatrick, T. J., Butzkueven, H., Tubridy, N., McDonald, E., & Galea, M. P. (2006). Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Multiple Sclerosis Journal, 12(5), 620–628.
Mori, S., Wakana, S., Zijl, P. C. M. V., & Nagae-Poetscher, L. M. (2005). MRI Atlas of Human White Matter. Elsevier.
Podsiadlo, D., & Richardson, S. (1991). The timed “up & go’’: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39(2), 142–148.
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., Filippi, M., Fujihara, K., Havrdova, E., Hutchinson, M., Kappos, L., Lublin, F. D., Montalban, X., O’Connor, P., Sandberg-Wollheim, M., Thompson, A. J., Waubant, E., Weinshenker, B., & Wolinsky, J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2), 292–302.
Rovaris, M., Bozzali, M., Iannucci, G., Ghezzi, A., Caputo, D., Montanari, E., Bertolotto, A., Bergamaschi, R., Capra, R., Mancardi, G. L., Martinelli, V., Comi, G., & Filippi, M. (2002). Assessment of normal-appearing white and gray matter in patients with primary progressive multiple sclerosis: A diffusion-tensor magnetic resonance imaging study. Archives of Neurology, 59(9), 1406–1412.
Sbardella, E., Tona, F., Petsas, N., Upadhyay, N., Piattella, M., Filippini, N., Prosperini, L., Pozzilli, C., & Pantano, P. (2015). Functional connectivity changes and their relationship with clinical disability and white matter integrity in patients with relapsing-remitting multiple sclerosis. Multiple Sclerosis Journal, 21(13), 1681–1692.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., Watkins, K. E., Ciccarelli, O., Cader, M. Z., Matthews, P. M., & Behrens, T. E. J. (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505.
Stafford, I. S., Kellermann, M., Mossotto, E., Beattie, R. M., MacArthur, B. D., & Ennis, S. (2020). A systematic review of the applications of artificial intelligence and machine learning in autoimmune diseases. Npj Digital Medicine, 3(1), 1–11.
Steenwijk, M. D., Daams, M., Pouwels, P. J. W., Balk, L. J., Tewarie, P. K., Killestein, J., Uitdehaag, B. M. J., Geurts, J. J. G., Barkhof, F., & Vrenken, H. (2014). What explains gray matter atrophy in long-standing multiple sclerosis? Radiology, 272(3), 832–842.
Tommasin, S., De Giglio, L., Ruggieri, S., Petsas, N., Giannì, C., Pozzilli, C., & Pantano, P. (2018). Relation between functional connectivity and disability in multiple sclerosis: a non-linear model. Journal of Neurology, 265(12), 2881–2892.
Tona, F., Petsas, N., Sbardella, E., Prosperini, L., Carmellini, M., Pozzilli, C., & Pantano, P. (2014). Multiple sclerosis: Altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology, 271(3), 814–821.
Tournier, J.-D., Smith, R., Raffelt, D., Tabbara, R., Dhollander, T., Pietsch, M., Christiaens, D., Jeurissen, B., Yeh, C.-H., & Connelly, A. (2019). Mrtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage, 202, 116137.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B., & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289.
Vapnik, V., & Chapelle, O. (2000). Bounds on error expectation for support vector machines. Neural Computation, 12(9), 2013–2036.
Veraart, J., Fieremans, E., & Novikov, D. S. (2016). Diffusion MRI noise mapping using random matrix theory. Magnetic Resonance in Medicine, 76(5), 1582–1593.
Veraart, J., Novikov, D. S., Christiaens, D., Ades-aron, B., Sijbers, J., & Fieremans, E. (2016). Denoising of diffusion MRI using random matrix theory. NeuroImage, 142, 394–406.
Wallin, M. T., Culpepper, W. J., Nichols, E., Bhutta, Z. A., Gebrehiwot, T. T., Hay, S. I., Khalil, I. A., Krohn, K. J., Liang, X., Naghavi, M., Mokdad, A. H., Nixon, M. R., Reiner, R. C., Sartorius, B., Smith, M., Topor-Madry, R., Werdecker, A., Vos, T., Feigin, V. L., & Murray, C. J. L. (2019). Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet Neurology, 18(3), 269–285.
Woo, C.-W., Chang, L. J., Lindquist, M. A., & Wager, T. D. (2017). Building better biomarkers: brain models in translational neuroimaging. Nature Neuroscience, 20(3), 365–377.
Yassa, M. A., & Stark, C. E. L. (2009). A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. NeuroImage, 44(2), 319–327.
Eitel, F., Soehler, E., Bellmann-Strobl, J., Brandt, A. U., Ruprecht, K., Giess, R. M., Kuchling, J., Asseyer, S., Weygandt, M., Haynes, J.-D., et al. (2019). Uncovering convolutional neural network decisions for diagnosing multiple sclerosis on conventional mri using layer-wise relevance propagation. NeuroImage: Clinical, 24, 102003.
Hartman, D., Hlinka, J., Paluš, M., Mantini, D., & Corbetta, M. (2011). The role of nonlinearity in computing graph-theoretical properties of resting-state functional magnetic resonance imaging brain networks. Chaos: An Interdisciplinary Journal of Nonlinear Science, 21(1), 013119.
He, X., Cai, D., & Niyogi, P. (2006). Laplacian score for feature selection. In Y. Weiss, B. Schölkopf, J. C. Platt (Eds.), Advances in Neural Information Processing Systems (Vol. 18, pp. 507–514). MIT Press.
Kocevar, G., Stamile, C., Hannoun, S., Cotton, F., Vukusic, S., Durand-Dubief, F., & Sappey-Marinier, D. (2016). Graph theory-based brain connectivity for automatic classification of multiple sclerosis clinical courses. Frontiers in Neuroscience, 10(478), 1–11.
Marzullo, A., Kocevar, G., Stamile, C., Durand-Dubief, F., Terracina, G., Calimeri, F., & Sappey-Marinier, D. (2019). Classification of multiple sclerosis clinical profiles via graph convolutional neural networks. Frontiers in Neuroscience, 13, 1–15.
Schoonheim, M. M., Meijer, K. A., & Geurts, J. J. G. (2015). Network collapse and cognitive impairment in multiple sclerosis. Frontiers in Neurology, 6, 1–5.
Schoonheim, M. M., Geurts, J. J. G., Barkhof, F., & Radiology and nuclear medicine, Pathology, Anatomy and neurosciences, NCA - Multiple Sclerosis and Other Neuroinflammatory Diseases, and NCA - Neurodegeneration. (2010). The limits of functional reorganization in multiple sclerosis. Neurology, 74(16), 1246–1247.
Tozlu, C., Jamison, K., Gauthier, S. A., & Kuceyeski, A. (2021). Dynamic functional connectivity better predicts disability than structural and static functional connectivity in people with multiple sclerosis. Frontiers in neuroscience, 15, 1–12.
Westin, C. F., Maier, S. E., Mamata, H., Nabavi, A., Jolesz, F. A., & Kikinis, R. (2002). Processing and visualization for diffusion tensor MRI. Medical Image Analysis, 6(2), 93–108.
Zurita, M., Montalba, C., Labbé, T., Cruz, J. P., Dalboni da Rocha, J., Tejos, C., Ciampi, E., Cárcamo, C., Sitaram, R., & Uribe, S. (2018). Characterization of relapsing-remitting multiple sclerosis patients using support vector machine classifications of functional and diffusion MRI data. NeuroImage: Clinical, 20, 724–730.
Funding
This work was supported by Ministry of Health of the Czech Republic – DRO (Kralovske Vinohrady University Hospital – FNKV, IN: 00064173, National Institute of Mental Health – NIMH, IN: 00023752, and Institute for Clinical and Experimental Medicine – IKEM, IN: 00023001), Charles University (112616/GAUK/2016, 260533/SVV/2022, Q35, Q37, Q41, Cooperatio - Neuroscience); the Czech Technical University Internal Grant Agency (SGS19/169/OHK3/3T/13, SGS22/062/OHK3/1T/13), the Czech Science Foundation project No. 13-23940S, Czech Health Research Council Project No. NU21-08-00432, the Czech Academy of Sciences Strategy AV21 Research Programmes “Hopes and Risks of the Digital Age” and “Breakthrough Technologies for the Future – Sensing, Digitisation, Artificial Intelligence and Quantum Technologies”, and by the long-term strategic development financing of the Institute of Computer Science (RVO:67985807) of the Czech Academy of Sciences.
Author information
Authors and Affiliations
Contributions
BRB: Methodology, Formal analysis, Writing - Original Draft, Visualization JM: Data Curation AS: Software, Data Curation JK: Software, JT: Investigation RAD: Conceptualization, KR: Conceptualization, Project administration, Funding acquisition, JH: Conceptualization, Project administration, Funding acquisition, Methodology, Supervision. All authors participated on editing the manuscript and approved its final version.
Corresponding author
Ethics declarations
Ethics approval
The study design was approved by the Ethics Committee of the Faculty Hospital Královské Vinohrady.
Consent to participate
All participants were informed about the experimental setup and provided written informed consent in accordance with the Declaration of Helsinki.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Tables 2, 3, 4, Figs. 5 and Tables 5, 6 and 7.
P-values of McNemar test between all thresholds and classifiers: Support Vector Machines (left), Logistic Regression (right). Threshold values in Support Vector Machines stand for the percentage of all features, in Logistic regression they represent the number of PCA component added to the model. FA – Fractional Anisotropy; FC – Functional Connectivity; GMV – Grey Matter Volume; all – combination of all three modalities
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rehák Bučková, B., Mareš, J., Škoch, A. et al. Multimodal-neuroimaging machine-learning analysis of motor disability in multiple sclerosis. Brain Imaging and Behavior 17, 18–34 (2023). https://doi.org/10.1007/s11682-022-00737-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00737-3