Abstract
Background
The etiology of degenerative supraspinatus tendon (SSP) tear is still subject to discussion.
Objectives
Our objective was to correlate clinical, radiological, and intraoperative signs of mechanical outlet impingement in patients with degenerative SSP tears.
Materials and methods
This prospective study included 100 patients with degenerative SSP tears that required surgery. Preoperatively, clinical impingement signs and radiological parameters (critical shoulder angle [CSA], acromion type according to Bigliani, acromion index [AI]) were recorded. Intraoperatively, the extent of the rupture and grinding marks on the bottom of the acromion were assessed.
Results
Of the 100 patients, 59 had clinical impingement signs preoperatively; 90 patients had at least one positive radiological sign (CSA > 35°, AI > 0.67, acromion type II or III). In 23 patients a partial tear, in 55 patients a full thickness tear, and in 22 patients an additional infraspinatus tendon tear were found. In 10 cases no grinding marks at the bottom of the acromion during arthroscopy were found. In 75 cases moderate grinding marks and in 15 cases severe marks with bare bone at the bottom of the acromion were found. There was no statistically significant correlation between preoperative impingement signs and arthroscopic grinding marks (p = 0.83) or between clinical signs and radiological parameters (p = 0.44). There was no significant correlation between extent of the rupture, extent of grinding marks or radiological impingement parameters (p = 0.16; p = 0.26).
Conclusion
We could not verify a correlation between clinical and radiological impingement sign and arthroscopic impingement parameters. Based on our study, degenerative SSP tear cannot be characterized as the result of a mechanical outlet impingement.
Zusammenfassung
Hintergrund
Die Ätiologie der degenerativen Supraspinatussehnen(SSP)-Ruptur ist immer noch Gegenstand der Diskussion.
Fragestellung
In dieser Studie wurde die Frage untersucht, ob es einen Zusammenhang zwischen klinischen, radiologischen und intraoperativen Zeichen eines mechanischen Outlet-Impingements bei Patienten mit degenerativer SSP-Ruptur gibt.
Material und Methodik
In dieser prospektiven Studie wurden 100 Patienten mit operationswürdiger SSP-Ruptur einbezogen. Präoperativ wurden klinische Impingementzeichen sowie radiologische Parameter (kritischer Schulterwinkel, CSA; Akromiontyp nach Bigliani; Akromion-Index, AI) erfasst. Intraoperativ wurden das Ausmaß der Ruptur (nach Bateman) sowie das Ausmaß der Schliffspuren an der Unterseite des Akromions erfasst.
Ergebnisse
Präoperativ wiesen 59 von 100 Patienten positive Impingementzeichen auf. Mindestens ein positives radiologisches Zeichen lag bei 90 Patienten vor (CSA > 35°, AI > 0,67; Akromiontyp II oder III). Bei 23 Patienten zeigte sich intraoperativ eine partielle SSP-Ruptur und bei 55 Patienten eine transmurale SSP-Ruptur. Eine 2‑Sehnen-Ruptur bestand bei 22 Patienten. In 10 Fällen waren keine Schliffspuren am Akromion sichtbar, in 75 Fällen moderate Schliffspuren, und in 15 Fällen lag der Knochen an der Akromionunterfläche frei. Es zeigte sich kein statistisch signifikanter Zusammenhang zwischen positiver Impingementsymptomatik und Schliffspuren am Akromion (p = 0,83) oder zwischen positiver Impingementsymptomatik und den radiologischen Parametern (p ≥ 0,44). Ein Zusammenhang zwischen Rupturgröße und Schliffspuren oder den radiologischen Parametern war ebenfalls nicht festzustellen (p = 0,16; p = 0,26).
Schlussfolgerung
Es ließ sich kein Zusammenhang zwischen klinischen und radiologischen Impingementzeichen und intraoperativen Befunden nachweisen. Daher lässt sich auf dem Boden der vorliegenden Ergebnisse die SSP-Ruptur nicht als Folge eines mechanisches Outlet-Impingements darstellen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
There is ongoing debate as to whether degenerative supraspinatus (SSP) tendon tears are associated with an impingement syndrome [13]. In addition, subacromial decompression often accommodates a surgical rotator cuff repair since less subacromial space is thought to be one reason for degenerative tears [1]. As a result, the number of arthroscopic acromioplasty interventions has risen significantly over the past 30 years [21, 22].
In general, there are two different theories concerning the etiology of degenerative SSP tears: In 1949 Armstrong [2] described for the first time a subacromial impingement as the cause of rotator cuff tears. Neer, in 1972 [16], also supported the theory that external factors, such as the acromion morphology, lead to contact between the SSP tendon and the acromion, which damages the tendon over time.
In 1931, Codman [9] postulated the theory of an intrinsic etiology. He described the decreasing vascularization and atrophy of the muscles with increasing age as the main cause of degenerative rotator cuff tears.
In addition, the term “impingement syndrome” as often used in the clinical setting includes several different pathologies. What Armstrong and Neer first described is what is currently known as a “mechanical outlet impingement” where structures that border the subacromial space change in a way that narrows the subacromial space. This is to be differentiated from the non-mechanical outlet impingement where enlargement of structures in the subacromial space, such as a bursitis, narrows the space. These two forms together are called “primary subacromial impingement.” Functional narrowing of the subacromial space due to muscular dysfunction and malalignment in the glenohumeral joint are called “secondary subacromial impingement.” In this study we focus on primary mechanical outlet impingement [10].
To date, few studies have investigated the etiology of degenerative rotator cuff tears and a possible correlation with the clinical signs of impingement syndrome. Most studies so far have concentrated on clinical or radiological findings when investigating degenerative SSP tears and mechanical outlet impingement. This is the first study that includes clinical and radiological impingement markers as well as arthroscopic findings and correlates these in patients with degenerative SSP tears.
We hypothesized that the degenerative SSP tendon tear is a mechanical outlet impingement lesion and there is a statistically significant correlation between radiological impingement markers, clinical sings of impingement, and arthroscopic findings in patients with degenerative SSP tendon tears to support this theory.
Patients and methods
We conducted our study according to the Declaration of Helsinki.
In this single-center, prospective case-series study, 100 consecutive patients with degenerative SSP tears that required arthroscopic repair of the supraspinatus tendon from February 2018 to May 2019 were included.
Exclusion criteria were traumatic tears, reactive shoulder stiffness, or additional significant lesion of the subscapularis tendon (> I° Fox and Romeo) or dislocation of the long biceps tendon.
All consecutive patients that fit the aforementioned clinical and intraoperative inclusion criteria were enrolled in the study. No patient was excluded in the course of this study.
Preoperative diagnostics including medical history, physical examination of the shoulder, radiographs of the shoulder in three planes, and magnetic resonance imaging (MRI) were conducted during routine examinations of patients. All patients signed written consent that their pre- and intraoperative data can be used for scientific purposes.
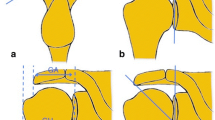
Clinical impingement signs were examined by the attending orthopedic physician during preoperative consultation using the following clinical tests: Neer, Hawkins–Kennedy, and the painful arc. The following radiological parameters were recorded: the critical shoulder angle, acromion morphology according to Bigliani, and the acromion index. Critical shoulder angle (CSA) and the acromion index (AI) were evaluated from an anterior–posterior (ap) radiograph of the shoulder. The acromion morphology was evaluated from a Y-view radiograph of the shoulder. Radiological parameters were evaluated by an orthopedic resident (JE). According to the study of Moor et al. [15], a CSA > 35° was defined as a positive radiological sign. According to the study of Nyffeler et al. [17], an increased AI (AI > 0.67) is associated with rotator cuff tears. Therefore, an acromion morphology type II and III and an AI > 0.67 were defined as positive radiological signs.
All 100 patients underwent arthroscopic rotator cuff repair with or without subacromial decompression. All surgeries were performed by the same senior surgeon (ML). The arthroscopic visualization of the inferior surface of the acromion was divided into three types according to an adjusted Copeland–Levy classification for arthroscopic evaluation of subacromial impingement [3]:
-
Type I: No grinding marks or minor grinding marks
-
Type II: Moderate grinding marks
-
Type III: Severe grinding marks with areas of bare bone
The extent of the rupture was also categorized (partial tear, full thickness tear, and involvement of the infraspinatus tendon).
The acquired data were tested for statistically significant correlation (p < 0.05) between either clinical or radiological impingement sign and arthroscopic acromion morphology using the chi-squared test. Statistically significant correlations between the extent of the tear and impingement parameters or acromion morphology were tested.
Results
The average patient age of the study population was 60 ± 8 years (range: 44–78 years). Of the patients, 21 were female and 79 were male. In 66 cases, the dominant side was affected.
In total, 59% of all patients showed at least one clinical impingement sign. The average CSA of all patients was 35.6 ± 3.94° (range 25–44°) and the average AI of all patients was 0.70 ± 0.08 (range 0.50–0.88). The most common acromion type in all tear groups was type II (64%). The results of registered clinical symptoms and radiological signs are displayed in Table 1.
Intraoperatively, ten patients showed no grinding marks, 75 patients showed moderate grinding marks, and 15 patients showed severe grinding marks with bare bone visible on the inferior surface of the acromion.
Overall, 23 patients had a partial tear, 55 patients had a full-thickness tear of the SSP, and 22 patients had a two-tendon tear involving the infraspinatus tendon (ISP), as verified by arthroscopy.
Relatively more patients with a partial tear had a type I acromion than did patients with a full-thickness or two-tendon tear, while relatively more of the latter patients had type III acromion. This tendency was not statistically significant (p = 0.90). Detailed results for radiological parameters according to the extent of the tear are given in Table 2.
Average CSA increased slightly from partial to two-tendon tears, whereas AI showed similar results for all tear sizes. There was no statistically significant difference in CSA or AI between patients with partial tear, patients with full-thickness tear of the SSP, and patients with a two-tendon tear (p ≥ 0.80). Positive radiological signs could not be identified preoperatively as an indicator of the extent of SSP tendon tear (p ≥ 0.25).
There was no significant correlation between radiological parameters and arthroscopic extent of grinding marks on the acromion (p = 0.26).
The correlation between preoperative impingement signs and arthroscopic grinding marks at the bottom of the acromion was not statistically significant (p = 0.83). The results of statistical tests for any positive clinical impingement signs as well as for every single clinical sign were not significant (Fig. 1). Therefore, preoperative positive clinical impingement signs could not be identified as predictors of the extent of grinding marks on the inferior surface of the acromion intraoperatively.
Furthermore, no statistically significant correlation between clinical impingement signs and the extent of the rupture were identified (p = 0.89; Fig. 2). The extent of rupture did not increase with increased number of positive impingement signs (p = 0.86).
Moderate grinding marks on the acromion were the most common arthroscopic finding for all rupture sizes (59–80%). In patients with two-tendon tears, bare bone on the inferior surface of the acromion was found more often than in patients with a partial or full-thickness SSP tear (32% vs. 9–11%, respectively). The correlation between the arthroscopic extent of grinding marks on the acromion and the extent of the SSP tendon tear was not statistically significant (p = 0.16; Fig. 3). Neither was there a significant correlation between clinical signs and radiological parameters (p = 0.44).
Discussion
In this study, no correlation was found between clinical impingement signs, radiological impingement markers in patients with SSP tears, and the intraoperative findings in the subacromial space.
The radiological parameters used in this study have been evaluated in patients with impingement syndrome and degenerative SSP tears in the literature. Studies in the literature report different results, from showing no significant difference to a healthy control [19] to studies that show significantly higher CSA and AI in patients with impingement syndrome and/or degenerative SSP tear [4, 5].
For example, Balke et al. [4] compared patients with degenerative and traumatic SSP tears and found a significantly higher CSA (36.8° ± 3.6 vs. 35.3° ± 2.9°) and AI (0.77 ± 0.07 vs. 0.73 ± 0.06) in patients with degenerative SSP tears. Their collective had a higher CSA and AI than ours did.
Overall, our patient collective had an average AI of 0.70 ± 0.08, which is above our cut-off point for a “pathological” AI (0.67) and higher than the healthy control groups described in the literature. A CSA between 30 and 35° is considered normal according to Moor et al. [15]. Our study population with degenerative SSP tears had a CSA just above this value with an average of 35.6°.
The literature on the influence of the acromion morphology according to Bigliani is controversial. Studies by Bigliani [7] and by MacGillivray [12] showed that a type III acromion is associated with an increased rate of rotator cuff tears. However, Ozaki et al. [18] could not find a statistically significant correlation.
One major problem using the Bigliani classification is the poor interobserver reliability [8].
We did not find a correlation between the arthroscopic findings of the acromion and clinical or radiological impingement markers or tear size. No study to date has compared all these parameters, but different studies have researched single aspects. In most studies, a statistically significant correlation between the arthroscopic visualization of subacromial grinding marks and the size or morphology of the SSP tear was found.
In a recent study by Miyake et al. [14], a significant correlation between subacromial damage in arthroscopy using the Copeland–Levy classification and size of the tendon tear was reported. This is contrary to our findings, but comparability is restricted since they categorized tear size differently than we did.
Kanatli et al. [11] compared the arthroscopic findings of patients with articular-sided partial thickness tears, which are not believed to be associated with impingement syndrome, and patients with bursal-sided partial thickness tears. Patients with articular-sided lesions mostly showed no or very little subacromial damage, whereas patients with bursal-sided lesions mostly showed little to moderate subacromial damage. The findings for the bursal-sided lesions were statistically significant. These findings support the hypothesis that the degenerative SSP tendon tear is an impingement lesion. In our study, we did not distinguish between bursal and articular partial-thickness tears, since we only had 23 patients with partial-thickness tears, and thus statistically significant results would be difficult to achieve.
Regarding radiological and clinical impingement parameters, there is no conclusive evidence to date that shows a correlation between arthroscopic findings and clinical and radiological impingement markers, which is consistent with our findings [14]. In the aforementioned study by Miyake et al. [14] no significant difference between the groups in CSA and AI were found.
Limitations
Our study has several limitations. We did not have a control group to compare our patients with. To conclusively evaluate a correlation between degenerative SSP tears and mechanical outlet impingement, a control group is needed. But intraoperative findings of patients without SSP tears are difficult to obtain because these patients seldomly undergo shoulder arthroscopy. We showed that there is no significant difference between varying tear sizes in terms of clinical symptoms, radiological markers, and arthroscopic findings. Taking into account the study by Balke et al. [5], which found no significant difference between patients with impingement syndrome and with SSP tear, we cannot rule out a significant difference in our studied parameters with either a healthy control group or patients with traumatic tears. Comparing the results of this study with patients who have traumatic SSP tears can represent future research work.
Radiographic and arthroscopic evaluation of parameters was only done by one researcher. A double-blind design could improve the accuracy of our results. Furthermore, CSA measurements show a good intra- and interobserver reliability but are very sensitive to changes in radiographic projections. Even deviations of 5° from a true ap projection can lead to more than 2° changes in the measured CSA [20].
We used the three most common clinical impingement tests for our study. But the literature does not report a high specificity for mechanical outlet impingement with these tests.
A review by Beaudreuil et al. [6] on clinical impingement signs showed a sensitivity of 75–89% for the Neer test and 91–92% for the Hawkins–Kennedy test. Specificity and positive predictive value were low. We did not find any statistically significant data on the painful arc.
Practical conclusion
Overall, there is still no unequivocal data as to whether the degenerative supraspinatus tear is a mechanical outlet impingement lesion.
The results of our study do not lend support to this theory.
Change history
02 June 2021
An Erratum to this paper has been published: https://doi.org/10.1007/s11678-021-00645-w
Abbreviations
- AI:
-
Acromion index
- ap:
-
Anterior–posterior
- CSA:
-
Critical shoulder angle
- ISP:
-
Infraspinatus
- MRI:
-
Magnetic resonance imaging
- SSP:
-
Supraspinatus
References
Abrams GD, Gupta AK, Hussey KE et al (2014) Arthroscopic repair of full-thickness rotator cuff tears with and without acromioplasty: randomized prospective trial with 2‑year follow-up. Am J Sports Med 42:1296–1303
Armstrong JR (1949) Excision of the acromion in treatment of the supraspinatus syndrome; report of 95 excisions. J Bone Joint Surg Br 31B:436–442
Atoun E, Gilat R, Van Tongel A et al (2017) Intraobserver and interobserver reliability of the Copeland-Levy classification for arthroscopic evaluation of subacromial impingement. J Shoulder Elbow Surg 26:2167–2172
Balke M, Liem D, Greshake O et al (2016) Differences in acromial morphology of shoulders in patients with degenerative and traumatic supraspinatus tendon tears. Knee Surg Sports Traumatol Arthrosc 24:2200–2205
Balke M, Schmidt C, Dedy N et al (2013) Correlation of acromial morphology with impingement syndrome and rotator cuff tears. Acta Orthop 84:178–183
Beaudreuil J, Nizard R, Thomas T et al (2009) Contribution of clinical tests to the diagnosis of rotator cuff disease: a systematic literature review. Joint Bone Spine 76:15–19
Bigliani LU, Ticker JB, Flatow EL et al (1991) The relationship of acromial architecture to rotator cuff disease. Clin Sports Med 10:823–838
Bright AS, Torpey B, Magid D et al (1997) Reliability of radiographic evaluation for acromial morphology. Skelet Radiol 26:718–721
Codman EA, Akerson IB (1931) The pathology associated with rupture of the supraspinatus tendon. Ann Surg 93:348–359
Hünnebeck SM, Balke M, Müller-Rath R et al (2020) Evidence-based recommendations for the treatment of mechanical outlet impingement. Obere Extrem 15:217–227
Kanatli U, Ayanoglu T, Aktas E et al (2016) Grade of coracoacromial ligament degeneration as a predictive factor for impingement syndrome and type of partial rotator cuff tear. J Shoulder Elbow Surg 25:1824–1828
Macgillivray JD, Fealy S, Potter HG et al (1998) Multiplanar analysis of acromion morphology. Am J Sports Med 26:836–840
Michener LA, Mcclure PW, Karduna AR (2003) Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech 18:369–379
Miyake S, Tamai M, Takeuchi Y et al (2020) Where and what damage occurs at the acromial undersurface in patients with rotator cuff tears? J Shoulder Elbow Surg. https://doi.org/10.1016/j.jse.2020.02.002
Moor BK, Bouaicha S, Rothenfluh DA et al (2013) Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: A radiological study of the critical shoulder angle. Bone Joint J 95-B:935–941
Neer CS 2nd (1972) Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am 54:41–50
Nyffeler RW, Werner CM, Sukthankar A et al (2006) Association of a large lateral extension of the acromion with rotator cuff tears. J Bone Joint Surg Am 88:800–805
Ozaki J, Fujimoto S, Nakagawa Y et al (1988) Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavera. J Bone Joint Surg Am 70:1224–1230
Sasiponganan C, Dessouky R, Ashikyan O et al (2019) Subacromial impingement anatomy and its association with rotator cuff pathology in women: radiograph and MRI correlation, a retrospective evaluation. Skelet Radiol 48:781–790
Suter T, Gerber Popp A, Zhang Y et al (2015) The influence of radiographic viewing perspective and demographics on the critical shoulder angle. J Shoulder Elbow Surg 24:e149–e158
Vitale MA, Arons RR, Hurwitz S et al (2010) The rising incidence of acromioplasty. J Bone Joint Surg Am 92:1842–1850
Yu E, Cil A, Harmsen WS et al (2010) Arthroscopy and the dramatic increase in frequency of anterior acromioplasty from 1980 to 2005: an epidemiologic study. Arthroscopy 26:S142–S147
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Engelke, M. Loew, S. Sulzer, S. Lichtenberg and M. Schnetzke declare that they have no competing interests.
We conducted our study according to the Declaration of Helsinki. The study was approved by the ethics committee of the ATOS clinic Heidelberg (06/2020).
Additional information
The original online version of this article was revised: Due to a technical problem the article was made retrospective open access.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Engelke, J., Loew, M., Sulzer, S. et al. Supraspinatus tear—a mechanical outlet impingement lesion?. Obere Extremität 16, 108–113 (2021). https://doi.org/10.1007/s11678-021-00628-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11678-021-00628-x