Abstract
Doubled haploid (DH) plants have been widely used for breeding and biological research in crops. Populus spp. have been used as model woody plant species for biological research. However, the induction of DH poplar plants is onerous, and limited biological or breeding work has been carried out on DH individuals or populations. In this study, we provide an effective protocol for poplar haploid induction based on an anther culture method. A total of 96 whole DH plant lines were obtained using an F1 hybrid of Populus simonii × P. nigra as a donor tree. The phenotypes of the DH population showed exceptionally high variance when compared to those of half-sib progeny of the donor tree. Each DH line displayed distinct features compared to those of the other DH lines or the donor tree. Additionally, some excellent homozygous lines have the potential to be model plants in genetic and breeding studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Populations of homozygous recombinant inbred lines (RILs) have been widely used for quantitative trait locus (QTL) mapping (Addison et al. 2021; Arif et al. 2021). However, RIL construction requires at least six generations to approach homozygosity using conventional inbreeding or selfing procedures. In contrast, doubled haploidy achieves homozygosity in one generation. Because of the ability to generate homozygosity in such a short time, doubled haploid (DH) populations have been used extensively in plant breeding, especially in maize. The DH lines of maize exhibit high genetic variance or display notable transgressive segregation for specific traits (Bordes et al. 2006; Mayor and Bernardo 2009). Given these unique attributes, DH populations are used for mapping QTLs, backcross breeding (Pillen et al. 2003), bulked segregant analysis (BSA) (Ardiel et al. 2002), construction of genetic maps, elite crossing, and genomic research (Seymour et al. 2012; Lee et al. 2016).
Poplars (the genus Populus) have strong adaptability, wide distributions, and the advantages of rapid growth and easy reproduction. Poplars are also the main afforestation tree species in the world today (Douglas 2017; Jha 2018). Black cottonwood (Populus trichocarpa Torr. & A. Gray ex Hook.) was the first forest species to have a complete genome sequence (Tuskan et al. 2006), and consequently, poplars have become the model woody plant to investigate the molecular biology of trees. Most forest trees have high levels of genetic diversity, presumably due to outcrossing and their large population sizes (Hamrick et al. 1981; Porth and El-Kassaby 2014). Consequently, most have a high genetic load of deleterious or lethal mutations, so inbreeding is greatly limited (Namkoong and Bishir 1987).
Unlike crops that are monoecious and could produce homozygotes by several generations of selfing, most poplars are dioecious, and induction of haploid material from parthenogenetic reproduction or microspore culture may be the most effective method for producing homozygous genomes. Various methods, such as cross-pollination with stressed pollen, heat-treated pollen (Winton and Einspahr 1968) or pollen treated with toluidine Winton blue (Illies 1974), have been used to induce haploid plants. However, anther culture with phytohormone treatment in tissue culture is the most often used method for generating haploids in poplar species (Deutsch et al. 2004; Li et al. 2013; Yang et al. 2017).
Although numerous studies have contributed to the induction methods for generating haploid plants, woody plants are usually recalcitrant to standard procedures. In the last twenty years, there have been no reports of the production of DH populations of woody plants. Therefore, unlike in a wide variety of other plants, little effort has been made to study the genetics and breeding of DH woody plants. In the present study, we constructed a DH population from an F1 hybrid of P. simonii × P. nigra and provide some novel findings. Our results should encourage studies of haploids in poplar and other forest tree species.
Materials and methods
Plant materials
The anther donor tree of P. simonii × P. nigra was located on the Northeast Forestry University campus in Harbin, China. Twigs with flower buds were cut from the donor tree, and bud scales were removed. Catkins were surface sterilized for 30 s in 70% alcohol and then 5 min in 3% sodium hypochlorite (12% available chlorine). The anthers were cultured in Petri dishes on full-strength Murashige and Skoog (MS) medium (Murashige and Skoog 1962) and kept in a light-proof box in a plant growth room until callus derived from the anthers could be tested. The medium was supplemented with 2 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D), 1 mg L−1 kinetin, and 3 g L−1 Gelrite. Calluses of haploid origin were transferred to differentiation medium to induce adventitious shoots. When the shoots were approximately 2.0 cm tall, they were detached from the callus and transferred to a rooting medium containing 0.2 mg L−1 1-naphthaleneacetic acid (NAA). The growth room was maintained at 25 °C under a 16-h light/8-h dark cycle. After 20 − 25 d, the rooted plantlets were transplanted into soil.
Flow cytometry test
The nuclei for flow cytometry (FCM) were obtained from anther callus, and the young leaves of the donor tree were dissociated by mechanical damage in a nuclear extraction buffer containing 0.004% (w/v) MgCl2·6H2O, 0.025% (w/v) NaCl, 0.0035% (w/v) EDTANa2·2H2O, 0.0095% (w/v) Na2S2O5, 0.0315 M Tris–HCl (pH = 7.0), 0.05% (w/v) PVP-10 and 0.5% (v/v) Triton X-100. Approximately 1 g of anther callus or young leaves of the donor tree was added to 1 mL of nuclear isolation buffer and rapidly chopped using double-sided blades. The suspension was then filtered through a 70 μm nylon filter to remove fragments and large tissue debris. Nuclei were collected by centrifugation (1000 rpm, 8 min), resuspended in 500 μL of propidium iodide (PI) and chilled at 4 °C for 20 min. Samples were put into the flow cytometer, and data were collected for 20,000 cells with the same flow rate, voltage and dye and analyzed for ploidy.
Next-generation sequencing and k-mer analysis
Total genomic DNA was extracted from callus or plantlet leaves using the cetyl trimethyl ammonium bromide (CTAB) method (Porebski et al. 1997). DNA fragment size and homogeneity were visualized on 1% agarose gels, and quantification and absorbance were monitored using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Library construction and whole-genome sequencing were performed by the Beijing Genomic Institute (BGI, Wuhan, China) on the MGISEQ-2000 platform. To obtain high-quality reads, fastp (version 0.19.7) was used to filter raw reads, and FastQC (version 0.11.8) used to check the quality of the clean reads (Chen et al. 2018). The k-mer analysis and genome assessment were performed using Genomic Character Estimator (GCE, version 1.0.2) (Liu et al. 2013). The k-mer distribution was obtained using Excel 2019. The primers (1534-F: 5′-CGTGTAGCACTAGATGCCCAATTCAATC-3′, 1534-R: 5′-GCCTCCTCGTCACCTCTAAAGAAACC-3′) were used for the amplification of the flanking sequence of the SNP site, and the primers (1534-Alignment-F: 5′-GCCTTTGCACTTCTTTTGCC-3′, 1534-Alignment-R: 5′-TTGGAATTAGAAGTGGGC-3′) were used to perform Sanger sequencing alignment of the SNP site.
Genetic variation in tree height and basal diameter of DH clones and the F2 generation
Tree height (TH) and basal diameter (BD) of 49 annual F2 families and 7 DH lines were investigated. DH16, DH109, DH127, DH158, DH171, DH212 and DH316 were represented by 40, 13, 51, 53, 18, 16 and 15 individuals, respectively. The TH and BD were measured directly using a tape measure and a Vernier caliper. All data were analyzed using SPSS version 25.0. Family heritability was estimated as follows:
where, F was derived from analysis of variance.
The phenotypic coefficient of variation (PCV) was calculated using the formula:
where, X and SD are the phenotypic mean and standard deviation of the trait, respectively.
Phenotypic correlation analysis of trait x and trait y was calculated according to the following formula:
where, Covp12 was the phenotypic covariance between traits, and \({{\upsigma }^{2}}_{\mathrm{p}1}\) and \({{\upsigma }^{2}}_{\mathrm{p}2}\) were the phenotypic variances for trait x and trait y, respectively.
Selected single guide (sg) RNA and analysis of mutant cell lines
A gene editing system was established using CRISPR‒Cas9 (Ueta et al. 2017). Agrobacterium tumefaciens-mediated transformation of DH callus line L23 was performed as described for tobacco (Nicotiana tabacum L.) BY-2 cells (An 1985). A genomic DNA fragment of PsnPDS was PCR amplified with gene-specific primers (PsnPDS-F: 5’-ATGAGTGCATTGAACTTGAGCTGG-3’, PsnPDS-R: 5’-TTAAGTAATGGTTGCCTCAGTCAAC-3’) designed based on its homologous target gene (Potri.014g148700.8) sequence in Populus trichocarpa. According to the obtained genome sequence and coding mRNA sequence, the 20 bp sequence containing a protospacer adjacent motif (PAM) structure was selected as the sgRNA. The sequence was close to the 5′ end, and the GC content was greater than 40%. For detection of the mutation in the PsnPDS gene in the transgenic L23 cell line, genomic DNA was extracted from a stable transgenic and wild-type (WT) cell line following a typical CTAB extraction protocol. The genomic DNA extracted from the L23 cell line was then used as a template to amplify the endogenous PDS fragment by PCR. PCR was performed using specific primers for the target (PsnPDS-detect-F: 5’-TTAGAGGCAGTGAATCAATGGGC-3’, PsnPDS-detect-R: 5’-TATCGCACAATCTCTCAAGAGTACATG-3’). The PCR product was separated on a 1% agarose gel, and bands were recovered and cloned into the pEASY-T1 vector (TransGen Biotech, Beijing, China). The mutations were identified by Sanger sequencing of individual clones. All sequence results were compared with the reference sequence of the PsnPDS gene by alignment in BioEdit (Hall 1999).
Results
Haploid callus induction
The results show that when the anther bears microspores mostly in the mid-uninucleate and late-uninucleate stages (Figs. 1A, B), the haploid callus induction rate is the highest, and it is almost impossible to obtain haploid callus when microspores are after the late-uninucleate stage. Numerous potential haploid calluses were obtained from poplar anthers after five weeks of culture on induction medium (Fig. 1C). Finally, 330 callus lines of haploid origin were obtained, and the positive rate of haploid origin of all the calluses was approximately 10% (330/3340). The callus lines showed significant differences in growth rate, dispersibility (friability) and morphology, implying that there is significant genetic variance in the callus population (Figs. 1D − F).
Induction of haploid callus. A State of the floral stage used for anther culture. Bar = 0.5 cm. B DAPI staining of mid-to-late-uninucleate microspores. Bar = 20 µm. C The callus was obtained after anther culture for 5 weeks. Red arrows show callus regenerated from anthers. D Different haploid callus lines showed significant variance in morphology. 1–9: Haploid callus lines L2, L19, L34, L35, L65, L69, L91, L96, and L219. E Haploid callus line L158 showed a quick growth rate on solid hormone medium with a tenfold increase in biomass after culture for two weeks. Bar = 1 cm. F Haploid callus line L44 showed obvious rapid mortality. Bar = 1 cm
DH whole-plant population displayed a variety of phenotypes
To obtain whole plants, 116 callus lines of haploid origin were transferred to adventitious shoot-inducing medium under a 16-h light/8-h dark cycle at 25 °C for plant regeneration through organogenesis. When the adventitious shoots were two centimeters in length, they were transferred to rooting medium to generate complete plantlets (Fig. 2A). The rooted plantlets were transferred into soil for continued growth (Fig. 2B). Flow cytometry (FCM) tests showed that all the plantlet lines were DHs, suggesting that there was spontaneous chromosome doubling during the development of adventitious shoots and that haploid poplar plantlets survived at extremely low rates. Finally, 96 callus lines successfully regenerated to whole plants, at which point 20 haploid callus lines had failed to induce shoots.
DH plantlet regeneration and a variety of phenotypes. A Adventitious shoot induced from haploid callus. B Two-year-old DH plants cultured in soil. C DH21 showed a different bark morphology compared to that of the donor control after grafting for 3 years. D The stems of DH4 and DH5 plants showed distinct variation compared to that of the donor control. The transverse section indicates toluidine blue staining. Bar = 200 µm. E and F Two-year-old DH92 and DH171 showed a shrub-like morphology
After being planted in soil, most lines exhibited slow and weak growth compared with that of the control donor tree (Fig. 2B). The DH plants grown under field conditions showed exceptionally high genetic variance. Almost every plant line growing in soil exhibited at least one distinct morphological trait compared to that of other DH lines or the donor tree, such as leaf and stem shape (Figs. 2C − F) or other characters. Some lines such as DH92 and DH171 showed a shrub-like morphology (Figs. 2E, F). The genetic variance in plant height of an F2 population from the half-sib progeny of the donor tree was compared to that of the DHs, and the latter showed significantly higher genetic variance (Table 1). Under the same growth conditions, most DHs possessed weak resistance to biotic or abiotic stress such as drought and infection by spider mites or pathogenic bacteria and viruses. Some plant lines were successfully cultured under field conditions for more than two years.
DH plant potential for multiple applications in genetic and breeding research
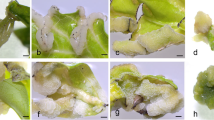
The DH plants showed homozygous genomes which are useful for genetic or breeding studies. To date, no Populus DH plant line has been widely used as a popular tool in research. Efforts were made to identify ideal DH lines over the past several years. Finally, we found that some DH lines that showed excellent potential as model plants. The cells of several callus lines are highly susceptible to Agrobacterium tumefaciens, and we performed knockout of the phytoene desaturase gene (PDS) with the CRISPR-Cas9 system using L23 line. The transgenic lines showed an albinism phenotype of callus under light culture conditions, suggesting that there was successful knockout of the PDS gene (Figs. 3A–C). We suggest that L23 may be an ideal tool for molecular biological studies. Although most DH lines showed low vigor and a high mortality rate under field conditions due to viral infection or other stresses, some individual lines showed a high survival rate. Some DH21 plants survived for three years under field conditions (Fig. 3D), and this line was previously used in a molecular genetic study (Liu et al. 2021). DH158 depicted ~ 100% survival after two years of cultivation and showed a high growth rate of 2 m in one year of culture (Fig. 3E). Thus, it has the potential to be an excellent candidate for cross breeding in the future.
Haploid potential for multiple applications. A − C CRISPR/Cas9-mediated gene editing in the transgenic L23 cell line. (A) Acquisition of kanamycin-resistant callus after culture for 4 weeks. B − C Phenotypic observation of the transgenic strain when callus was cultured under light conditions. psnpds: mutant strain; WT: Wild type. D DH21 plant (marked with a red arrow) that survived for three years under field conditions. E DH158 showed a high growth rate of 2 m in one year of culture
Protocol for confirmation of haploid origin
Although anther culture is a very effective way to produce haploid poplar plants, an efficient protocol for identifying callus with a haploid origin is not available. In previous studies, simple sequence repeat (SSR) analysis was used for genome homozygosity testing (Deutsch et al. 2004; Yang et al. 2017). However, the design of SSR marker primers and the SSR analysis procedure are time-consuming. To effectively identify calluses with a haploid origin, a simple improved-throughput protocol was established by combining FCM with the SNP identification method. Haploid callus lines were first obtained by determining the ploidy level using FCM (Fig. 4A). For the diploid and other polyploid lines, we checked for genome homozygosity by direct DNA sequencing of PCR products of SNP sites in the donor tree (Fig. 4B). In the present study, the results of de novo genome sequencing were screened, and two SNP sites selected. Forty-five callus lines of haploid origin were surveyed as determined by direct DNA sequencing of PCR products and performed k-mer analysis. Finally, we proved that our method showed 100% accuracy for each SNP site (Fig. 4C). This protocol was effective, as we were able to check more than 500 callus lines per day. The process for DH plant induction described in the present study is shown in Fig. 5.
Confirmation of haploid origin. A DNA ploidy level test using FCM. 1, Anther donor tree. 2, Haploid callus line L21. 3, Anther donor tree and haploid callus line merge. B Detection of heterozygosity of anther callus by SNP site. 1, Anther donor tree. 2, Doubled haploid whole plant of line DH158. 3, Haploid callus line L29. 4, Haploid callus line L171. C Distribution of 17-mer frequency. 1, Anther donor tree. 2, Doubled haploid whole plant of line DH158. 3, Haploid callus line L29. 4, Haploid callus line L171
Discussion
Heterozygosity of a plant genome can create difficulties in genomic research because, to a variable extent, a heterozygote represents two genome sequences that need to be analyzed simultaneously. For example, both RNA interference (RNAi) and CRISPR technology require that the guiding RNA be highly specific to the target gene sequence, and heterozygosity could reduce specific base pairing and seriously affect the efficiency of gene suppression (Gitlin et al. 2002; Zhou et al. 2015). Although poplars have been used as model woody plants for studying the molecular biology of trees, most have high genome heterozygosity (Ingvarsson and Bernhardsson 2020; Huang et al. 2021). Haploid induction would be the most effective method to achieve homozygosity. However, it is still difficult to obtain enough haploid individuals for poplar genetic research. In the present study, an efficient system for obtaining DH lines through poplar anther culture was developed, and 96 DH whole-plant lines were obtained, representing the first report of the construction of a DH population of woody plants. Haploid and DH poplars will be ideal plant materials for RNAi and CRISPR technology because there should be essentially no SNPs in the genomes of poplars of haploid origin. Homozygosity of haploid and DH poplars will provide high-quality genome assemblies, similar to those of other haploid plants such as apple (Daccord et al. 2017) and rose (Raymond et al. 2018). Therefore, some haploid and DH lines were identified that have the potential to be ideal tools for molecular biology and breeding studies in the future. Our research will contribute to the protocol of haploid and DH plant induction in woody plants.
During the first meiotic division, homologous chromosomes pair and undergo reciprocal crossing over, which recombines linked sequence variation (Lawrence et al. 2017) and generates genetic diversity in haploid gametes so that each haploid product has a unique genetic composition. The DH lines of maize exhibited higher genetic variance than selfing lines (Bordes et al. 2006; Mayor and Bernardo 2009). In theory, the heterozygosity of poplar will result in higher genetic variance among haploids. However, few studies have focused on the genetic variation of haploid poplars because it is difficult to construct DH poplar populations. In this study, both the haploid callus and the DH whole-plant population from P. simonii × P. nigra exhibited very high genetic variance. Because haploid or DH plants carry only half of the chromosomes from their diploid parent, the recessive genes expressed in the donor tree lead to unusual phenotypes in DH plants. In this way, it becomes possible to observe the phenotypes of rare recessive alleles that have never before been observed. DHs can also lead to the identification of recessive lethal or semi lethal genes. Our results also suggested that DH populations would be useful for genome-wide selection for traits such as timber yield and horticultural traits in many important woody plants.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Addison CK, Angira B, Cerioli T, Groth DE, Richards JK, Linscombe SD, Famoso AN (2021) Identification and mapping of a novel resistance gene to the rice pathogen. Cercospora Janseana Theor Appl Genet 134(7):2221–2234. https://doi.org/10.1007/s00122-021-03821-2
An G (1985) High efficiency transformation of cultured tobacco cells. Plant Physiol 79(2):568–570
Ardiel GS, Grewal TS, Deberdt P, Rossnagel BG, Scoles GJ (2002) Inheritance of resistance to covered smut in barley and development of a tightly linked SCAR marker. Theor Appl Genet 104(2–3):457–464. https://doi.org/10.1007/s001220100696
Arif MAR, Shokat S, Plieske J, Ganal M, Lohwasser U, Chesnokov YV, Kocherina NV, Kulwal P, Kumar N, McGuire PE, Sorrells ME, Qualset CO, Börner A (2021) A SNP-based genetic dissection of versatile traits in bread wheat (Triticum aestivum L.). Plant J 108(4):960–976. https://doi.org/10.1111/tpj.15407
Bordes J, Charmet G, De Vaulx RD, Pollacsek M, Beckert M, Gallais A (2006) Doubled haploid versus S1 family recurrent selection for testcross performance in a maize population. Theor Appl Genet 112(6):1063–1072. https://doi.org/10.1007/s00122-006-0208-3
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34(17):i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Daccord N, Celton JM, Linsmith G, Becker C, Choisne N, Schijlen E, van de Geest H, Bianco L, Micheletti D, Velasco R, Di Pierro EA, Gouzy J, Rees JG, Guérif P, Muranty H, Durel CE, Laurens F, Lespinasse Y, Gaillard S, Aubourg S, Quesneville H, Weigel D, van de Weg E, Troggio M, Bucher E (2017) High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat Genet 49(7):1099–1106. https://doi.org/10.1038/ng.3886
Deutsch F, Kumlehn J, Ziegenhagen B, Fladung M (2004) Stable haploid poplar callus lines from immature pollen culture. Physiol Plant 120(4):613–622. https://doi.org/10.1111/j.0031-9317.2004.0266.x
Douglas CJ (2017) Populus as a model tree. In: Groover A, Cronk Q (eds) Comparative and evolutionary genomics of angiosperm trees. Springer, Berlin, pp 61–84
Gitlin L, Karelsky S, Andino R (2002) Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 418(6896):430–434. https://doi.org/10.1038/nature00873
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuclc Acids Symposium Series 41(41):95–98. https://doi.org/10.14601/Phytopathol_Mediterr-14998u1.29
Hamrick JL, Mitton JB, Linhart YB (1981) Levels of Genetic Variation in Trees: Influence of life history characteristics. Gen. Tech. Rep. PSW-GTR-48. Berkeley, CA: Pacific Southwest Forest and Range Exp. Stn, Forest Service, U.S. Department of Agriculture. pp. 35−41.
Huang X, Chen S, Peng X, Bae EK, Dai X, Liu G, Qu G, Ko JH, Lee H, Chen S, Li Q, Lu M (2021) An improved draft genome sequence of hybrid Populus alba × Populus glandulosa. J for Res 32:1663–1672. https://doi.org/10.1007/s11676-020-01235-2
Illies ZM (1974) Induction of haploid parthenogenesis in aspen by post-pollination treatment with Toluidine-blue. Silvae Genet 23:221–226
Ingvarsson PK, Bernhardsson C (2020) Genome-wide signatures of environmental adaptation in European aspen (Populus tremula) under current and future climate conditions. Evol Appl 13(1):132–142. https://doi.org/10.1111/eva.12792
Jha KK (2018) Biomass production and carbon balance in two hybrid poplar (Populus euramericana) plantations raised with and without agriculture in southern France. J for Res 29:1689–1701. https://doi.org/10.1007/s11676-018-0590-0
Lawrence EJ, Griffin CH, Henderson IR (2017) Modification of meiotic recombination by natural variation in plants. J Exp Bot 68(20):54711–55483. https://doi.org/10.1093/jxb/erx306
Lee GH, Kang IK, Kim KM (2016) Mapping of Novel QTL Regulating grain shattering using doubled haploid population in rice (Oryza sativa L.). Int J Genomics 2016:2128010. https://doi.org/10.1155/2016/2128010
Li Y, Li H, Chen Z, Ji LX, Ye MX, Wang J, Wang L, An XM (2013) Haploid plants from anther cultures of poplar (Populus × beijingensis). Plant Cell Tiss Organ Cult 114(1):39–48. https://doi.org/10.1007/s11240-013-0303-5
Liu B, Shi Y, Yuan J, Hu X, Zhang H, Li N, Li Z, Chen Y, Mu D, Fan W (2013) Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects. Quant Biol 35(s1–3):62–67. https://doi.org/10.1016/S0925-4005(96)02015-1
Liu B, Wang S, Tao X, Liu C, Qu G, Dou Q (2021) Molecular karyotyping on Populus simonii × P. nigra and the derived doubled haploid. Int J Mol Sci 22(21):11424. https://doi.org/10.3390/ijms222111424
Mayor PJ, Bernardo R (2009) Genomewide selection and marker-assisted recurrent selection in doubled haploid versus F2 populations. Crop Sci 49(5):1719–1725. https://doi.org/10.2135/cropsci2008.10.0587
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Namkoong G, Bishir J (1987) The frequency of lethal alleles in forest tree populations. Evolution 41(5):1123–1126. https://doi.org/10.1111/j.1558-5646.1987.tb05882.x
Pillen K, Zacharias A, Léon J (2003) Advanced backcross QTL analysis in barley (Hordeum vulgare L.). Theor Appl Genet 107(2):340–352. https://doi.org/10.1007/s00122-003-1253-9
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15(1):8–15. https://doi.org/10.1007/BF02772108
Porth I, El-Kassaby YA (2014) Assessment of the genetic diversity in forest tree populations using molecular markers. Diversity 6(2):283–295. https://doi.org/10.3390/d6020283
Raymond O, Gouzy J, Just J, Badouin H, Verdenaud M, Lemainque A, Vergne P, Moja S, Choisne N, Pont C, Carrère S, Caissard JC, Couloux A, Cottret L, Aury JM, Szécsi J, Latrasse D, Madoui MA, François L, Fu X, Yang SH, Dubois A, Piola F, Larrieu A, Perez M, Labadie K, Perrier L, Govetto B, Labrousse Y, Villand P, Bardoux C, Boltz V, Lopez-Roques C, Heitzler P, Vernoux T, Vandenbussche M, Quesneville H, Boualem A, Bendahmane A, Liu C, Le Bris M, Salse J, Baudino S, Benhamed M, Wincker P, Bendahmane M (2018) The Rosa genome provides new insights into the domestication of modern roses. Nat Genet 50(6):772–777. https://doi.org/10.1038/s41588-018-0110-3
Seymour DK, Filiault DL, Henry IM, Monson-Miller J, Ravi M, Pang A, Comai L, Chan SW, Maloof JN (2012) Rapid creation of Arabidopsis doubled haploid lines for quantitative trait locus mapping. Proc Natl Acad Sci 109(11):4227–4232. https://doi.org/10.1073/pnas.1117277109
Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, Schein J, Sterck L, Aerts A, Bhalerao RR, Bhalerao RP, Blaudez D, Boerjan W, Brun A, Brunner A, Busov V, Campbell M, Carlson J, Chalot M, Chapman J, Chen GL, Cooper D, Coutinho PM, Couturier J, Covert S, Cronk Q, Cunningham R, Davis J, Degroeve S, Déjardin A, Depamphilis C, Detter J, Dirks B, Dubchak I, Duplessis S, Ehlting J, Ellis B, Gendler K, Goodstein D, Gribskov M, Grimwood J, Groover A, Gunter L, Hamberger B, Heinze B, Helariutta Y, Henrissat B, Holligan D, Holt R, Huang W, Islam-Faridi N, Jones S, Jones-Rhoades M, Jorgensen R, Joshi C, Kangasjärvi J, Karlsson J, Kelleher C, Kirkpatrick R, Kirst M, Kohler A, Kalluri U, Larimer F, Leebens-Mack J, Leplé JC, Locascio P, Lou Y, Lucas S, Martin F, Montanini B, Napoli C, Nelson DR, Nelson C, Nieminen K, Nilsson O, Pereda V, Peter G, Philippe R, Pilate G, Poliakov A, Razumovskaya J, Richardson P, Rinaldi C, Ritland K, Rouzé P, Ryaboy D, Schmutz J, Schrader J, Segerman B, Shin H, Siddiqui A, Sterky F, Terry A, Tsai CJ, Uberbacher E, Unneberg P, Vahala J, Wall K, Wessler S, Yang G, Yin T, Douglas C, Marra M, Sandberg G, Van de Peer Y, Rokhsar D (2006) The genome of black cottonwood, Populus trichocarpa (Torr & Gray). Science 313(5793):1596–1604. https://doi.org/10.1126/science.1128691
Ueta R, Abe C, Watanabe T, Sugano SS, Ishihara R, Ezura H, Osakabe Y, Osakabe K (2017) Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci Rep 7(1):507. https://doi.org/10.1038/s41598-017-00501-4
Winton LL, Einspahr DW (1968) The use of heat-treated pollen for aspen haploid production. For Sci 14(4):406–407. https://doi.org/10.1093/forestscience/14.4.406
Yang J, Li K, Li C, Li J, Zhao B, Zheng W, Gao Y, Li C (2017) In vitro anther culture and Agrobacterium-mediated transformation of the AP1 gene from Salix integra Linn. in haploid poplar (Populus simonii × P. nigra). J for Res 29(2):321–330. https://doi.org/10.1007/s11676-017-0453-0
Zhou X, Jacobs TB, Xue LJ, Harding SA, Tsai CJ (2015) Exploiting SNPs for biallelic CRISPR mutations in the outcrossing woody perennial Populus reveals 4-coumarate:CoA ligase specificity and redundancy. New Phytol 208(2):298–301. https://doi.org/10.1111/nph.13470
Acknowledgements
We are grateful to professor Changbin Chen for his productive discussion.
Funding
This work was supported by the National Key R&D Program of China (2021YFD2200203), Heilongjiang Province Key R&D Program of China (GA21B010), Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team) and Heilongjiang Postdoctoral Financial Assistance (LBH-Z21097).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was supported by the National Key R&D Program of China (2021YFD2200203), Heilongjiang Province Key R&D Program of China (GA21B010), Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team) and Heilongjiang Postdoctoral Financial Assistance (LBH-Z21097).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yu Lei.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, C., Wang, S., Liu, Y. et al. Exceptionally high genetic variance of the doubled haploid (DH) population of poplar. J. For. Res. 34, 1941–1950 (2023). https://doi.org/10.1007/s11676-023-01612-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-023-01612-7