Abstract
To explore differences in leaf morphology between Sapindus mukorossi and Sapindus delavayi, and how the environment might drive these differences, 80 germplasm samples from the Sapindus germplasm nursery in Fujian Province were selected. The study revealed a wide variation and diversity in 16 germplasm traits, both within and between species grown under the same conditions. On average, the relative contribution of intraspecific variability to total variability was more important (83%) than the relative contribution of interspecific variability (17%). PERMANOVA analysis showed differences in leaflet thickness, length, perimeter, length to width ratio, and leaf hairs or trichome density. Correlation analyses between leaf morphological traits and environmental variables indicated that leaves tended to be larger, longer, and thicker in wetter, warmer, and low-altitude conditions. Our analysis of the relationship between climate and leaf morphology revealed that S. mukorossi had a greater sensitivity to climate variation, particularly in response to mean temperatures of the coldest and warmest seasons, which led to differences in leaf traits and the distribution of the two species. These findings contribute to the understanding of leaf morphology variations in S. mukorossi and S. delavayi, and provide a basis for the collection of Sapindus germplasm resources, their cultivation and use to help address climate change.



Note Pairwise comparisons are a color gradient denoting Spearman’s correlation coefficient. Only results that had statistical significance (P < 0.05) are in color, and ‘***’ represents P < 0.01. Lon longitude, Lat latitude, Al altitude; Bio1 annual mean temperature, Bio2 mean diurnal range, Bio3 isothermality, Bio4: temperature seasonality, Bio7 temperature annual range, Bio8 mean temperature of wettest quarter, Bio9 mean temperature of driest quarter, Bio10 mean temperature of warmest quarter, Bio11 mean temperature of coldest quarter, Bio15 precipitation seasonality, Bio16 precipitation of wettest quarter, Bio17 precipitation of driest quarter, Bio18 precipitation of warmest quarter, Bio19 precipitation of coldest quarter, Bio20 solar radiation; LL leaflet length, LW leaflet width, LA leaflet area, LS leaflet shape index, LP leaflet perimeter, L/w leaflet length to width ratio, LSL rachis length, LT leaflet thickness, HD leaflet epidermal hair density, LPC petiolule color, LLC leaflet number; LOD leaflet overlap degree; LC leaflet color, LG foliar luster; LV leaflet venation

Note Pairwise comparisons of environmental factors are in color denoting Spearman’s correlation coefficient; leaf trait distribution was related to each environmental factor by partial Mantel tests. Edge width corresponds to the Mantel’s r statistic, and edge color denotes the statistical significance based on 9999 permutations

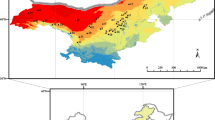
Note Green vectors point to the direction of the increase for a given variable so that germplasms with similar environmental variable profiles and morphological traits are localized in similar positions in the diagram. Bio10 mean temperature of warmest quarter, Bio11 mean temperature of coldest quarter, Bio18 precipitation of warmest quarter
Similar content being viewed by others
References
Anderson MJ, Walsh DC (2013) PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr 83(4):557–574
Bickford CP (2016) Ecophysiology of leaf trichomes. Plant Biol 43(9):807–814
Cao LM, Xia NH (2008) Structural characters of leaf epidermis and their systematic significance in Sapindacea from China. Acta Bot Yunnanica 30(04):405–421
Cao LM, Wang ZX, Cao M, Liu JH, Lin Q, Xia NH (2014) Leaf venation and its systematic significance in Sapindaceae of China. Plant Divers Resour 36(04):419–432
Chartzoulakis K, Patakas A, Kofidis G, Bosabalidis A, Nastou A (2002) Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Sci Hortic 95(1):39–50
Chitwood DH, Sinha NR (2016) Evolutionary and environmental forces sculpting leaf development. Curr Biol 26(7):297–306
Delectis F (1985) Delectis Florae Republicae Popularis Sinicae Agendae Academiae Sinicae Edita, 1985. Flora Republicae Popularis Sinicae, vol 47. Science Press, Beijing
Diao SF, Shao WH, Chen T, Jiang JM, Duan WB (2016) Gentic diversity of Sapindus mukorossi natural populations in China based on ISSR. For Res 29(02):176–182
Glennon KL, Cron GV (2015) Climate and leaf shape relationships in four Helichrysum species from the Eastern Mountain Region of South Africa. Evol Ecol 29(5):657–678
Guo Y, Xie JP, Liu AH, Hu XG, Luo YP, Wu ZQ, Bao FK (2011) Overview of the pharmacological action of Sapindus mukorossi. J Pathog Biol 6(11):873–874
Jung V, Albert CH, Violle C, Kunstler G, Loucougaray G, Spiegelberger T (2014) Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J Ecol 102(1):45–53
Koksheeva I, Kislov D, Tvorogov S, Doudkin R (2017) Relationships between leaf shape and climate in Rhododendron mucronulatum. Nord J Bot 35(5):618–626
Leigh A, Sevanto S, Ball MC, Close JD, Ellsworth DS, Knight CA, Nicotra AB, Vogel S (2012) Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol 194(2):477–487
Li AM, Lv ML, Zhou CM (2018) Phenotypic diversity analysis of leaf traits in cultivated Houttuynia cordata Thunb. populations from Hunan Province. Plant Sci J 36(1):73–85
Li HG, Chen DZ, Xu JS, Liu GJ, Pang XD, Ye JH, Mo XW, Chen HH (2019) Phenotypic diversity and variation in natural populations of Erythrophleum fordii, an endangered plant species. Sci Silvae Sin 55(04):69–83
Liu YB, Wang G, Liu J, Zhao X, Tan HJ, Li XR (2007) Anatomical, morphological and metabolic acclimation in the resurrection plant Reaumuria soongorica during dehydration and rehydration. J Arid Environ 70(2):183–194
Liu Y, Li X, Chen G, Li M, Liu M, Liu D (2015) Epidermal micromorphology and mesophyll structure of Populus euphratica heteromorphic leaves at different development stages. PLoS ONE 10(9):e0137701. https://doi.org/10.1371/journal.pone.0137701
Liu JM, Sun CW, He QY, Jia LM, Weng XH, Yu JP (2017) Research progress in Sapindus L. germplasm resources. World For Res 30(6):12–18
Mahar KS, Rana TS, Ranade SA, Meena B (2011) Genetic variability and population structure in Sapindus emarginatus Vahl from India. Gene 485(1):32–39
Mahar KS, Rana TS, Ranade SA, Pande V, Palni LMS (2013) Estimation of genetic variability and population structure in Sapindus trifoliatus L., using DNA fingerprinting methods. Trees 27(1):85–96
Mahar KS, Palni LMS, Ranade SA, Pande V, Rana TS (2017) Molecular analyses of genetic variation and phylogenetic relationship in Indian soap nut (Sapindus L.) and closely related taxa of the family Sapindaceae. Meta Gene 13:50–56
McKee M (2017) How does temperature impact leaf size and shape in four woody dicot species? Testing the assumptions of leaf physiognomy-climate models. Honors Theses—All 1744. https://wesscholar.wesleyan.edu/etd_hon_theses/1744
Monteiro MV, Blanuša T, Verhoef A, Hadley P, Cameron RWF (2016) Relative importance of transpiration rate and leaf morphological traits for the regulation of leaf temperature. Aust J Bot 64(1):32–44
Peppe DJ, Royer DL, Cariglino B, Oliver SY, Newman S, Leight E, Enikolopov G, Fernandez-Burgos M, Herrera F, Adams JM, Correa E, Currano ED, Erickson JM, Hinojosa LF, Hoganson JW, Iglesias A, Jaramillo CA, Johnson KR, Jordan GJ, Kraft NJB, Lovelock EC, Lusk CH, Niinemets Ü, Peñuelas J, Rapson G, Wing SL, Wright IJ (2011) Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol 190(3):724–739
Ragamustari SK, Sukara E (2019) Strengthening the genetic diversity conservation narrative in Indonesia: challenges and prospects. Biodivers Conserv 28(7):1647–1665
Rashidi F, Jalili A, Babaie Kafaki S, Sagheb-Talebi K, Hodgson J (2012) Anatomical responses of leaves of Black Locust (Robinia pseudoacacia L.) to urban pollutant gases and climatic factors. Trees 26(2):363–375
Royer DL (2012) Climate reconstruction from leaf size and shape: new developments and challenges. Paléontol Soc Pap 18:195–212
Royer DL, McElwain JC, Adams JM, Wilf P (2008) Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol 179(3):808–817
Sun ZZ, Li QY, Wang XK, Zhao WT, Xue Y, Feng JY, Liu XF, Liu MY, Jiang D (2017) Comprehensive evaluation and phenotypic diversity analysis of germplasm resources in Mandarin. Sci Agric Sin 50(22):4362–4383
Sun CW, Wang LC, Liu JM, Zhao GC, Gao SL, Xi BY, Duan J, Weng XH, Jia LM (2018) Genetic structure and biogeographic divergence among Sapindus species: an inter-simple sequence repeat-based study of germplasms in China. Ind Crop Prod 118:1–10
Sun CW, Jia LM, Xi BY, Liu JM, Wang LC, Weng XH (2019) Genetic diversity and association analyses of fruit traits with microsatellite ISSRs in Sapindus. J For Res 30(1):193–203
Swarnavalli GCJ, Dinakaran S, Raman N, Jegadeesh R, Pereira C (2017) Bio inspired synthesis of monodispersed silver nano particles using Sapindus emarginatus pericarp extract—study of antibacterial efficacy. J Saudi Chem Soc 21(2):172–179
Wright IJ, Dong N, Maire V, Prentice IC, Westoby M, Díaz S, Gallagher RV, Jacobs BF, Kooyman R, Law EA (2017) Global climatic drivers of leaf size. Science 357(6354):917–921
Wu H, Wang N, Weng Z, Xu DP, Wang HY, Yao WR (2013) Researches on surface activity of Sapindus saponin product and its mixed system. Fine Chem 30(02):149–154
Xu KJ, CiDan ZX, Ding LS (2013) Research progress on chemical constituents and biological activities of Sapindus Species. Nat Prod Res Dev 25(2):267–273, 257
Xu ZP, Shao ZT, Zhu XJ, Wang F, Wang YY, Xiao M, Ma KP (2019) Big Data portal development in agrobiodiversity: current research and future outlooks. J Agric Big Data 1(2):76–87
Yan XF, Zhao HK, Liu XD, Li QY, Wang YM, Yuan CP, Dong YS (2014) Phenotypic traits and diversity of different leaf shape accessions of the wild soybean (Glycine soja Sieb. et Zucc.) in China. Can J Plant Sci 94(2):397–404
Zhang CQ, Ji ZF, Lin LL, Zhao RH, Wang YL (2015) Phenotypic diversity of Acer mono maxim population. Acta Ecol Sin 35(16):5343–5352
Zhang TJ, Chen XH, Kang XK, Liu J (2019) Phenotypic diversity of leaf morphologic traits of Davidia involucrata natural populations in Sichuan Province. Acta Ecol Sin 38(01):35–43
Acknowledgements
We are grateful to the team of Yuanhua Forestry Biotechnology Co., Ltd., for providing the forestry materials and for assistance, and textcheck (http://www.textcheck.com/text/page/index) for polishing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was supported by the Fundamental Research Funds for the Central Universities of China (2015ZCQ-LX-02 2017-LYSJWJ-1).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yanbo Hu.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, J., Rui, X. et al. Biogeographic divergence in leaf traits of Sapindus mukorossi and Sapindus delavayi and its relation to climate. J. For. Res. 32, 1445–1456 (2021). https://doi.org/10.1007/s11676-020-01206-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01206-7