Abstract
Background
Quantifying intra-specific variation in leaf functional traits along environmental gradients is important for understanding species' responses to climate change. In this study, we assessed the degree of among and within populations variation in leaf functional traits and explored leaf response to geographic and climate change using Caryopteris mongholica as material, which has a wide range of distribution environments.
Results
We selected 40 natural populations of C. mongholica, measured 8 leaf functional traits, analyzed the extent of trait variation among and within populations, and developed geographic and climatic models to explain trait variation between populations. Our results showed that the variation in leaf functional traits of C. mongholica was primarily lower within populations compared to among populations. Specifically, the leaf area (LA) exhibited higher variability both among and within populations, whereas leaf carbon content (LC) exhibited lower variation within populations but greater variation among populations. We observed a specific covariation pattern among traits and a strong linkage between morphological, economic, and mechanical traits. Increasing minimum temperature, precipitation of month, and seasonal precipitation differences all limited the growth and development of C. mongholica. However, it was observed that an increase in mean annual precipitation positively influenced the morphological development of its leaf.
Conclusions
These results demonstrate the response of intra-specific trait variation to the environment and provide valuable insights into the adaptation of intra-specific leaf functional traits under changing climatic conditions.
Similar content being viewed by others
Introduction
As global climate change accelerates, extreme weather events are becoming more common, posing a significant threat to plant survival and growth [1]. Leaf functional traits, such as leaf shape, specific leaf area, and leaf nitrogen content, exhibit a high level of sensitivity and responsiveness to environmental changes. Consequently, these traits can serve as reliable indicators of plant responses to climate change [2, 3]. Studies have shown that the survival strategies of plants and their capacity to utilize resources are closely linked to leaf functional traits [4, 5].
Plant leaf functional traits are subject to considerable variation in response to climate change and often exhibit some intra-specific variation [6, 7], resulting from a combination of genetic variation and phenotypic plasticity [8]. In addition, leaf traits show varying degrees of variation both within and among populations. For example, in Swiss subalpine grasslands, leaf nutrient concentrations vary significantly among populations [9]; European beech also shows substantial variation in leaf size, SLA, and Huber value among populations [10]; Warren's study revealed significant variation in leaf morphology and physiology of red ironwood eucalyptus among and within populations [11]; Similarly, Xu's research revealed significant differences in leaf functional traits among populations of Cunninghamia lanceolata [12]. Additionally, the majority of results indicating greater among populations variation than within populations variation at larger study scales [12,13,14].
The growth traits of plant exhibit significant variation due to the influence of environmental factors [15, 16]. The functional traits of leaves are sensitive to environmental factors, such as water, radiation, and temperature, which can be adjusted to achieve optimal utilization of limiting resources and enhance plant viability [17]. Leaf size and shape are considered morphological traits that play a crucial role in determining water use efficiency and the amount of light intercepted for photosynthesis [18, 19]; Mechanical traits, such as dry matter content (LDMC) and carbon content (LC), provide insights into a plant's resistance to physical injury [20]. Specific leaf area (SLA), nitrogen content (LN) and phosphorus content (LP) are considered economic traits that reflect a plant's utilization and adaptation to environmental factors, as well as nutrient stability and limitations [21]. For example, leaves under drought and low-temperature conditions often display smaller size, lower specific leaf area, and increased leaf nitrogen content, which can improve water utilization and enhance photosynthetic efficiency [22,23,24].
However, the relationship between leaf traits and environmental factors is not fixed and can vary depending on the scale of the study. Furthermore, when environmental changes occur, plant traits often undergo simultaneous changes, rather than singularly, resulting in complex synergistic relationships among plant functional traits that collectively regulate and sustain plant life activities. For instance, in arid regions characterized by low precipitation and high evapotranspiration rates, woody plants have adapted to drought conditions by reducing transpiration through smaller leaf areas and higher tissue density compared to plants in other habitats at the same latitude [25]. However, studies have also indicated that in arid regions, the connection between plant leaf traits and environmental variables tends to diminish [26]. Consequently, the relationship between traits and environmental variables may not be clearly defined at different scales of study in arid and semi-arid regions. Understanding the drivers of plant trait variation and the interrelationships among traits in this region is crucial for accurately characterizing plant functional diversity in global carbon-climate models.
The current research on plant functional traits has predominantly focused on comparing the average trait values among species at large scales [27], which has overlooked the importance of intra-specific trait variation in maintaining species coexistence and community dynamics. There is growing evidence that intraspecific trait variation has a significant and non-negligible impact on species identity and ecosystem function due to phenotypic plasticity and local adaptation. Therefore, to advance our understanding of environmental effects on trait variation and predict species responses to climate change, it is essential to quantify intra-specific trait variation along environmental gradients. This is particularly important for widely distributed plants [28,29,30].
Caryopteris mongholica, a shrub species classified under the genus Caryopteris in the family Labiatae, is currently endangered. It primarily occupies arid and semi-arid regions [31] and plays a significant role in sand fixation, soil conservation, the retardation of desertification, and the stabilization of native ecological environments. Being the northernmost species within the Caryopteris genus, it holds a critical phylogenetic position [32]. Moreover, its population distribution encompasses a wide range of environmental conditions and exhibits substantial variation [33]. In response to anticipated future climate changes, its distribution area is gradually shifting northward [34]. Consequently, it serves as a valuable resource for studying intra-specific leaf trait variation within the species. However, the existing studies on C. mongholica are predominantly confined to limited geographical areas, and there is a lack of comprehensive comparisons of functional traits on broader scales. This limitation restricts our depth of understanding regarding phenotypic differentiation and ecological adaptation in this species. Therefore, in this study, we used C. mongholica as material to assess the degree of variation among populations and within populations in leaf functional traits across its range and explore leaf responses to geographic and climate change. We sampled C.mongholica leaves from various regions during July–September 2018–2021 and made the following hypotheses: (1) The functional traits of leaves in C.mongholica exhibit varying degrees of variation both within and among populations within its distribution range, and there are covariation patterns among these traits. (2) Each functional trait of leaf within the species demonstrates a response to changes in geographic gradients. (3) Each functional trait of leaf within the species adjusts its survival strategy in response to changes in climate. Testing these hypotheses will enhance our understanding of how environmental factors influence plant ecological strategies.
Materials and methods
Study area
We selected 40 representative populations of C. mongholica (Fig. 1) based on the natural population distribution provided by the China National Specimen Resources Sharing Platform (http://www.nsii.org.cn/2017/home.php), These populations were located at least 25 km apart and covered the primary natural geographical distribution area of C. mongholica in northern China. The 40 sample sites were mainly located in northwestern China (36°06′N—45°39′N and 96°21′E—116°45′E), which experiences a typical temperate continental arid climate with relatively low precipitation (annual mean precipitation 67—475 mm), strong temperature heterogeneity (annual mean temperature 1.03—9.59 °C), and a large altitude span (911—2805 m). The specific locations of the 40 sample sites are shown in Table S1.
Sample collection and determination
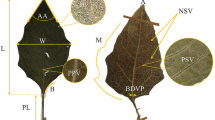
Between July and August of each year from 2018 to 2021, we established three 10 m × 10 m survey sample squares in each survey area with a spacing of at least 50 m between each square. Within each square, We selected ten healthy plants and collected 30–50 mature and morphologically intact leaves from each plant. The leaves were then scanned using a flatbed scanner (CanonScan LiDE 120, Canon, RoHS, WEEE), and the scanned images were analyzed using Image-Pro Plus 6.0 (Olympus, Tokyo, Japan) to obtain leaf morphological parameters. After weighing, the leaves were dried at 75 °C to a constant weight, and we calculated the specific leaf area of each sample from the corresponding leaf weight and area. Next, we ground the leaves and passed them through a 100-mesh sieve to determine their carbon, nitrogen, and phosphorus content. The carbon and nitrogen content of the samples were measured using a combined C/N analyzer (2400II CHNS/O, Element Analyzer, Perkin-Elmer, Boston, MA, United States), the determination of the phosphorus content was carried out using a molybdenum-antimony anti-colorimetric method [35].
Finally, we obtained a total of 8 trait indices, including leaf area (LA), perimeter (LPE), aspect ratio (LRA), specific leaf area (SLA), dry matter content (LDMC), carbon (LC), nitrogen (LN) and phosphorus (LP) content per unit mass, for 40 natural populations of C. mongholica.
Data sources and processing
Degree of variation and covariation in leaf functional traits
The variability of leaf traits within and among 40 populations of C. mongholica was investigated using SPSS 17.0 (SPSS Inc, Chicago, USA). The coefficient of variation (CV) for each of the 8 leaf traits within each population was calculated and averaged across all populations to assess the degree of trait variation within populations. For trait variation among populations, the degree of variation was quantified using two metrics: Phenotypic dissimilarity (PhD) index [36] and multiplicity of variation. In addition, we defined the leaf trait spaces using Principal Component Analysis (PCA) on imputed trait data by using the funspace function in the ‘funspace’ R package [37], resulting in the extraction of two trait principal components (T-PC1 and T-PC2). Furthermore, correlation analysis was performed to examine the relationships between the traits (corresponding to the first question).
Relationship between leaf traits and geographical gradients (longitude, latitude, altitude)
Meteorological data were acquired from WorldClim (http://www.worldclim.org/version2) with a spatial resolution of 30 s, and bioclimatic data for the sample plots were obtained through ArcGIS 10.6 software. A total of 17 climate variables were extracted, but after removing highly correlated variables (r > 0.90; Table S2), eight climate factors remained. Eight factors included five temperature variables and three precipitation variables (Table S3). Principal component analysis was used to reduce the dimensions of the data, resulting in the extraction of two principal components (E-PC1 and E-PC2), which accounted for 73.49% of the total variance. E-PC1 explained 49.24% of the total variance, the minimum temperature of the coldest month and mean temperature of the driest quarter had higher loadings on E-PC1. E-PC2 explained 24.25% of the total variance, and the mean annual precipitation had higher loadings on E-PC2. The correlation analysis revealed that E-PC1 was significantly correlated with longitude, latitude, and altitude, while E-PC2 was significantly correlated with longitude and altitude (p < 0.01, Table S3).
The effects of longitude, latitude, and altitude on the two principal component axes (T-PC1 and T-PC2) of the trait were analyzed using linear mixed model from the lme4 package of R 3.6.2, sites were treated as random effect (corresponding to the second question). After controlling for the effects of the two climatic principal components, we performed partial correlation analysis to identify the unique associations between geographic variables and leaf traits. For the visualization and prediction of each leaf functional trait, Kriging interpolation was utilized, and the data were examined to exclude the corresponding second-order trends and transformations. The predictions were made for the provinces where C. mongholica was distributed in the study area, this prediction process was carried out using ArcGIS 10.6.
Relationship between leaf traits and climate
Linear mixed model from the lme4 package was used to examine the relationship between leaf traits and climate variables (corresponding to the third question). Two sets of models were implemented: one considering the first two principal components (E-PC1 and E-PC2) of climate variables, and the other including all eight climate variables. The analysis was performed in R version 3.6.2.
Method statement
We ensured that we have permission to collect C. mongholica, the plant collection and investigation was approved by the Academy of Forestry Science, the Inner Mongolia Autonomous Region, China. The samples were carefully identified by Professor Meng Ji of Academy of Forestry Science and Professor Weilun Yin at Beijing Forestry University based on the descriptions in Flora of China, a voucher specimen was deposited in the Herbarium of Plant Biology Department, Beijing Forestry University with an accession number BJFU-CM117.
Results
Degree of variation in leaf functional traits
Leaf traits exhibited varying levels of among and within populations variability across the 40 sample areas. The within populations variability of 8 leaf functional traits in the wild population of C. mongholica was generally lower than the among populations variability. Within populations variation coefficients for leaf traits ranged from 4.28% to 17.53%, while among populations PhD index ranged from 9.21% to 22.54%. The largest variations were observed within and among populations for LA. On the other hand, LC exhibited minimal variation within populations but greater variation among populations. LRA showed less variation both within and among populations. All traits exhibited a variance variation of more than onefold between populations, and there were significant differences in leaf functional traits among populations (Table 1).
PCA analysis of leaf functional traits revealed that the eight traits could be categorized into two dimensions (T-PC1 and T-PC2). The combined contribution of these two principal component axes accounted for 63.91% of the total variance. Among the traits, LPE, LA, SLA, and LDMC exhibited larger vector lengths on the T-PC1 axis, while LRA and LP showed larger vector lengths on T-PC2. Additionally, traits exhibited a discernible covariance pattern across the three aspects of morphology, economy, and mechanical (Fig. 2).
Towards a leaf trait space. The trait space is defined by a PCA on leaf trait data. Colors indicate the probabilistic distribution of trait combinations in the functional trait space defined by a PCA (red = high probability; yellow = low probability). Contour lines indicate 0.95, 0.50, and 0.25 quantiles of the probability distribution. The output shows that there one hotspot. The variance explained by each component and the loadings of the original traits are also shown. LL, LPE, LRA (morphological traits); SLA, LN, LP (economic traits); LDMC, LC (mechanical traits)
Correlation analysis (Fig. S1) confirmed significant positive correlations (p < 0.01) between leaf morphological traits (LPE, LA, LRA) and negative correlations (p < 0.01) between morphological traits and mechanical traits (LDMC, LC). Additionally, significant positive correlations (p < 0.01) were observed among economic traits (SLA, LN, LP). Furthermore, SLA exhibited significant positive correlations (p < 0.05) with morphological traits and negative correlations (p < 0.05) with mechanical traits. However, there were no significant correlations between LRA, mechanical traits, and economic traits. In summary, the analysis showed that C. mongholica exhibited general correlations among leaf traits, with close relationships among morphological, mechanical, and economic traits.
Effect of geography on leaf traits
The linear mixed model analysis revealed a significant correlation between the T-PC1 axis of the traits and longitude (p < 0.05), while latitude and altitude did not show significant correlations with the two PC axes of the trait (Table 2). Regression analysis further confirmed a significant correlation between longitude and T-PC1 axis (Fig. S2). The results of the partial correlation analysis revealed that latitude showed a significant correlation with LC only, while longitude exhibited significant correlation with LPE, LA, LDMC, LC, and SLA. Additionally, altitude showed a significant correlation with LDMC under uncontrolled climate conditions (Table 3). Both the linear mixed models and the partial correlation analysis consistently indicated that leaf traits of C. mongholica were primarily influenced by longitude.
The distribution patterns of leaf traits were predicted and visualized using interpolation techniques (Fig. 3). The predictions indicated that the morphological traits (LPE and LA) of leaves exhibited a similar trend, gradually decreasing from southeast to northwest. Moreover, the LRA demonstrated a gradual increase from south to north within the range of 95°E-120°E, suggesting a pronounced bias towards ovoid growth in leaves located further south in this range. Furthermore, the SLA of C. mongholica leaves located east of 107°E was significantly higher than that of leaves located west of 107°E. LN and LP displayed a distribution pattern characterized by higher values in the central region and lower values on both sides, delineated by sample site 19. Similarly, LDMC was also divided by 107°E, with higher LDMC in the west compared to the east. West of 107°E, LDMC was centered around sample site 18 (39.79°N, 103.41°E) and gradually decreased in all directions. LC was centered around sample site 18 and showed a gradual decrease in all directions.
After controlling for hydrothermal conditions (E-PC1 and E-PC2), the significant correlations between leaf traits and latitude and longitude disappeared (Table 3). Regarding the relationship between LC-latitude, the significant correlation disappeared when controlling the influence of E-PC1. However, significant correlation remained when controlling the influence of E-PC2 (Table 3). Therefore, the patterns of LC along the latitudinal gradient were more influenced by temperature (represented by E-PC1) than precipitation (represented by E-PC2). Regarding the trait-longitude relationship, significant correlation with LDMC remained when controlling the influence of E-PC1, while significant correlations with LPE, LA, SLA, and LC disappeared. When controlling the influence of E-PC2, significant correlations with LA and LC still existed, but significant correlations with LPE, LDMC, and SLA disappeared. All significant correlations disappeared when controlling E-PC1 and E-PC2 simultaneously (Table 3). Thus, regarding the seven leaf traits along the longitude gradient, LA, and LC were more influenced by temperature (represented by E-PC1), while LDMC was more influenced by precipitation (represented by E-PC2), LPE and SLA were influenced by both climate variables (represented by E-PC1 and E-PC2). Regarding the altitude-trait relationship, only LDMC was significantly influenced by altitude, and along the altitude gradient, LDMC was more influenced by the climatic variables represented by E-PC2 than E-PC1 (Table 2).
Effect of climate on leaf traits
The results obtained from the linear mixed model analysis revealed important relationships between leaf traits and climate variables. Specifically, E-PC1 exhibited a significant negative correlation with LA and a significant positive correlation with LC. Similarly, E-PC2 exhibited a significant negative correlation with LDMC, while displaying significant positive correlations with LPE, LRA, and SLA (Table 4A). Temperature and precipitation were found to exert substantial effects on leaf traits (Table 4B). Among the eight climate factors, five were found to be the strongest predictors of leaf trait variation (Table 4B). These influential climate factors include temperature seasonality (BIO2), minimum temperature of coldest month (BIO4), annual precipitation (BIO10), precipitation of driest month (BIO12), and precipitation seasonality (BIO13). Specifically, BIO2 demonstrated a significant negative effect on LRA and LC, while the BIO4 displayed a significant negative effect on LPE and LRA, and a positive effect on LDMC. Moreover, annual precipitation (BIO10) exhibited a significant positive effect on LPE and a significant negative effect on LC. Additionally, precipitation of driest month (BIO12) exerted a significant negative effect on LPE and LA, but a significant positive effect on LC. Furthermore, the precipitation seasonality (BIO13) was found to have a significant negative effect on LPE and LRA, while showing a significant positive effect on LC. It is worth noting that SLA and LDMC were significantly influenced by E-PC2, representing precipitation, although the impact of a single climate factor was not significant.
Discussion
The 8 functional traits of C. mongholica leaves showed varying degrees of intra-specific variation both among and within populations, with within populations coefficients of variation ranging from 4.28%-17.53% and among populations coefficients of variation ranging from 9.21%-22.54% (Table 1). The variation was greater among populations than within population (Table 1). Microhabitat and genetic effects typically influence trait variation within populations [38], while geographic and climatic factors influence trait variation among populations [39,40,41]. In our study, C. mongholica leaf traits may be more influenced by geography and climate.
In this study, both within and among populations, leaf area exhibited the highest degree of variation. Under natural conditions, leaf size is a crucial trait that determines plant water use efficiency and photosynthetic efficiency [19, 42]. Furthermore, leaf size is highly responsive to environmental changes and possesses greater phenotypic plasticity [43]. Second, the LP was also more variable within and among populations. The LP of C. mongholica in this study was slightly higher than the national average for terrestrial plants (1.46 g/kg) [44]. It has been confirmed that the soil phosphorus content in most parts of China is lower than the global average, resulting in an overall low LP in plants [44], indicating that plant growth is more likely to be phosphorus-limited. Additionally, the variability of soil phosphorus content in China is increasing from the humid zone to the arid/semi-arid zone with a large overall variation [45], which may also explain the high variability of plant leaf LP observed in this study. In contrast, LC exhibited lower variation within populations but greater variation among populations. This may be attributed to the fact that carbon is a skeletal element that constitutes the plant, providing stability and support. As a result, LC within a given region tends to remain relatively consistent [46]. However, differences in environmental conditions, such as climate and soil, between populations can lead to variations in carbon content among populations.
Functional traits are not independent of each other and are naturally selected to form an optimal combination of traits that can adapt to specific environments [47, 48]. In this study (Fig. 2), we found significant correlations between morphological traits (LPE, LA, LRA) of C. mongholica leaves were significantly positively correlated with each other. Meanwhile, two mechanical traits (LDMC, LC) were significantly and negatively correlated with morphological traits. In addition, we found significant positive correlations between specific SLA, LN, and LP, positive correlation between SLA and morphological traits, as well as the negative correlation between SLA and mechanical traits (LDMC, LC), is in line with the global pattern of plant leaf trait correlations [49]. However, correlations between LN and LP with morphological and mechanical traits were less significant. Overall, there is a specific pattern of covariation among leaf traits in C. mongholica that adapts to the more heterogeneous northwestern arid habitat by adopting different trait combinations, with distributional trade-offs between structural toughness and rapid growth.
In this study, it was observed that almost all leaf traits of C. mongholica exhibited variation with geographic and climatic variables. Although the LN and LP were not significantly correlated with geographic and climatic factors, they were significantly correlated with SLA, which itself was influenced by climatic factors (Table 4). Therefore, it can be inferred that climatic factors indirectly influence leaf LN and LP by affecting SLA.
On the spatial gradient (Fig. 3), the morphological traits (LPE, LA) of C. mongholica leaves exhibit a gradual decrease from southeast to northwest of China. This trend is consistent with the variation observed in the average leaf size of woody plants in China, as reported by Li et al. [50]. Specifically, in warm and humid regions, plants tend to have larger leaves, while in arid and cold regions, plants typically exhibit smaller leaves. However, LRA demonstrated a tendency to become progressively larger with latitude from north to south. Plants in response to the environment with increased precipitation, by changing leaf shape (Fig. 3) and becoming progressively more rounded to facilitate water and air exchange with the outside world [51]. Both SLA and LDMC displayed different trends on both sides of 107°E, which suggests that factors beyond precipitation and temperature might be influencing these variations. In close proximity to the 107°E longitude lie the north–south Helan Mountains and the north–south flowing bend of the Yellow River. The presence of these mountains and the distribution of Yellow River's water resources may explain the differences in leaf traits between the two sides. The pattern of leaf traits along the spatial gradient is primarily influenced by climate (Table 3). The relationship between climate and leaf traits is complex, and changes in leaf functional traits cannot be predicted by a single climate factor (Table 3).
The outcomes derived from the analysis conducted using a linear mixed model (Table 4B) demonstrated that certain climatic variables, namely the BIO4, BIO12, and BIO13, exhibited a negative impact on leaf size (LPE, LA). Conversely, an increase in annual precipitation (BIO10) was found to positively contribute to the development of leaves. When exposed to extremely cold and dry conditions, C. mongholica produced smaller leaves due to the increased minimum temperature, increased precipitation of driest month, and increased seasonal variation in precipitation. The coldest month (January) and driest month (December) are both in winter, and deciduous plants adjust their metabolism to adapt to the winter season, which affects leaf development in the next growing season. Li et al. [52] employed Maxent software to forecast the potential habitat of C. mongholica in China. The study's results demonstrated that C. mongholica exhibited a low water requirement for its growth. We used Maxent to predict the distribution of C. mongholica in the fitness zone. The results showed that the survival of C. mongholica was severely limited in the coldest months when the minimum temperature was greater than -18 °C and the minimum rainfall was greater than 2 mm (Fig. S3). C. mongholica exhibits some cold tolerance and fear of flooding during winter to adapt to the dry and cold environment.
In this study, we observed that LDMC and LC, although displaying covariation, do not exhibit identical responses to geographical and climatic variables. LDMC is primarily influenced by longitude, while LC is mainly influenced by latitude. Among the climatic variables examined, LDMC exhibited a significant negative correlation with E-PC2, which primarily represents precipitation. On the other hand, LC displayed a significant positive association with E-PC1, representing temperature. Additionally, LC was found to be significantly influenced by multiple individual climatic factors. Consequently, it is plausible to speculate that although LDMC and LC exhibit a covariance pattern in trait variation, this pattern could be attributed to variations in other traits. Additionally, it should be noted that other local environmental factors that were not considered in this study, such as soil fertility, irradiation, and species richness, could also influence plant traits significantly. These factors have been significantly correlated with leaf traits in previous studies [53,54,55]. Currently, there is a debate regarding the relative strength of climate-dominant factors, such as temperature and precipitation, on leaf trait variation [56, 57]. Nevertheless, this study highlights that the variation in leaf functional traits within populations is driven by a combination of temperature and precipitation.
Conclusions
The functional traits of C.mongholica leaves exhibit varying degrees of trait variation within and among populations. In general, the within population variation tends to be smaller compared to the among population variation, and specific pattern of covariation among traits. Variation in most leaf traits is influenced by climatic conditions along a geographic gradient, with longitude having a stronger influence than latitude and altitude on leaf trait variation. Temperature and precipitation, in combination, significantly impact leaf trait variation, with temperature seasonality (BIO2), minimum temperature of coldest month (BIO4), annual precipitation (BIO10), precipitation of driest month (BIO12), and precipitation seasonality (BIO13) having a greater impact. Increasing minimum temperature, precipitation in the driest month, and seasonal variation in precipitation limit the growth and development of C. mongholica. Moreover, morphological traits, mechanical traits, and economic traits are intricately interconnected and regulate the trait development of leaves.
Availability of data and materials
The plant materials were collected from natural population in geographic distribution of C. mongholica. The datasets generated during the current study has been deposited in the Science Data Bank repository (https://www.scidb.cn). Data access link: https://cstr.cn/31253.11.sciencedb.07946. https://doi.org/10.57760/sciencedb.07946. All data generated during the current study are included in this published article.
Abbreviations
- LA:
-
Leaf area
- LPE:
-
Leaf perimeter
- LRA:
-
Leaf aspect ratio
- SLA:
-
Specific leaf area
- LDMC:
-
Leaf dry matter content
- LC:
-
Leaf carbon content
- LN:
-
Leaf nitrogen content
- LP:
-
Leaf phosphorus content
- CV:
-
Coefficient of variation
- PhD:
-
Phenotypic dissimilarity
- PCA:
-
Principal component analysis
- BIO1:
-
Annual Mean Temperature
- BIO2:
-
Temperature Seasonality (standard deviation *100)
- BIO3:
-
Maximum Temperature of Warmest Month
- BIO4:
-
Minimum Temperature of Coldest Month
- BIO7:
-
Mean Temperature of Driest Quarter
- BIO10:
-
Annual Precipitation
- BIO12:
-
Precipitation of Driest Month
- BIO13:
-
Precipitation Seasonality (Coefficient of Variation)
References
Zandalinas SI, Fritschi FB, Mittler R. Global warming, climate change, and environmental pollution: recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021;26(6):588–99. https://doi.org/10.1016/j.tplants.2021.02.011.
Wang H, Wang R, Harrison SP, Prentice IC. Leaf morphological traits as adaptations to multiple climate gradients. J Ecol. 2022;110(6):1344–55. https://doi.org/10.1111/1365-2745.13873.
Tsukaya H. Leaf shape diversity with an emphasis on leaf contour variation, developmental background, and adaptation. Semin Cell Dev Biol. 2018;79:48–57. https://doi.org/10.1016/j.semcdb.2017.11.035.
Fritz MA, Rosa S, Sicard A. Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front Genet. 2018;9:478. https://doi.org/10.3389/fgene.2018.00478.
Dong N, Prentice IC, Wright IJ, Evans BJ, Togashi HF, Caddy-Retalic S, et al. Components of leaf-trait variation along environmental gradients. New Phytol. 2020;228(1):82–94. https://doi.org/10.1111/nph.16558.
Henn JJ, Buzzard V, Enquist BJ, Halbritter AH, Klanderud K, Maitneret BS, et al. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front Plant Sci. 2018;9:1548. https://doi.org/10.3389/fpls.2018.01548.
Albert CH, Thuiller W, Yoccoz NG, Soudant A, Boucher F, Saccone P, et al. Intraspecific functional variability: extent, structure and sources of variation. J Eco. 2010;98:604–13. https://doi.org/10.1111/j.1365-2745.2010.01651.x.
Matesanz S, Horgan-Kobelski T, Sultan SE. Phenotypic plasticity and population differentiation in an ongoing species invasion. PLoS ONE. 2012;7:44955. https://doi.org/10.1371/journal.pone.0044955.
Firn J, Nguyen H, Schütz M, Risch AC. Leaf trait variability between and within subalpine grassland species differs depending on site conditions and herbivory. Proc Biol Sci. 1907;2019(286):20190429. https://doi.org/10.1098/rspb.2019.0429.
Weithmann G, Schuldt B, Link RM, Heil D, Hoeber S, John H, et al. Leaf trait modification in European beech trees in response to climatic and edaphic drought. Plant Biol (Stuttg). 2022;24(7):1272–86. https://doi.org/10.1111/plb.13366.
Warren CR, Tausz M, Adams MA. Does rainfall explain variation in leaf morphology and physiology among populations of red ironbark (Eucalyptus sideroxylon subsp. tricarpa) grown in a common garden? Tree Physiol. 2005;25(11):1369–78. https://doi.org/10.1093/treephys/25.11.1369.
Xu R, Cheng SD, Zhou J, Tigabu M, Ma XQ, Li M. Intraspecific variations in leaf functional traits of Cunninghamia lanceolata provenances. BMC Plant Biol. 2023;23(1):92. https://doi.org/10.1186/s12870-023-04097-y.
Lu YF, Pei NC, Zhu YJ, Bai ZL, Yang AN, Zhang JH, et al. Community structure and leaf trait diversity in a vulnerable species, Phoebe chekiangensis (Lauraceae). Chi J Appl Eco. 2018;29(07):2101–10. https://doi.org/10.13287/j.1001-9332.201807.013.
Ai Z, Xu TT, Zhou ZN, Ma F. Leaf morphological trait variations in natural populations of Caragana microphylla. Acta Bot Boreal. 2020;40(09):1595–604. https://doi.org/10.7606/j.issn.1000-4025.2020.09.1595.
Hansen AJ, Neilson RP, Dale VH, Flather CH, Iverson LR, Currie DJ, et al. Global change in forests: responses of species, communities, and biomes: interactions between climate change and land use are projected to cause large shifts in biodiversity. Bioscience. 2001;51:765–79. https://doi.org/10.1641/0006-3568(2001)051[0765:GCIFRO]2.0.CO;2.
Rowland L, Oliveira RS, Bittencourt PRL, Giles AL, Coughlin I, Costa PB, et al. Plant traits controlling growth change in response to a drier climate. New Phytol. 2021;229(3):1363–74. https://doi.org/10.1111/nph.16972.
Michaletz ST, Weiser MD, McDowell NG, Zhou JZ, Kaspari M, Helliker BR, et al. The energetic and carbon economic origins of leaf thermoregulation. Nature Plants. 2016;2:16129. https://doi.org/10.1038/nplants.2016.129.
Xu F, Guo WH, Xu WH, Wei YH, Wang RQ. Leaf morphology correlates with water and light availability: what consequences for simple and compound leaves? Prog Nat Sci. 2009;2009(19):1789–98. https://doi.org/10.1016/j.pnsc.2009.10.001.
Zhang S, Zhang Y, Ma K. The association of leaf lifespan and background insect herbivory at the interspecific level. Ecology. 2016;98:425–32. https://doi.org/10.1002/ecy.1649.
Cornelisse JHC, Lavorel S, Garnier E. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot. 2003;51:335–80. https://doi.org/10.1071/bt02124.
Ma RT, Fang Y, An SS, Zhao JF, Xiao L. Ecological stoichiometric characteristics of leaves and litter of plants dominant in Heidaigou opencast coal mining area. Acta Pedol Sin. 2016;53:1003–14. https://doi.org/10.11766/trxb201512200490.
Han S, Chen SM, Song AP, Liu RX, Li HY, Jiang JF, et al. Photosynthetic responses of Chrysanthemum morifolium to growth irradiance: morphology, anatomy and chloroplast ultrastructure. Photosynt. 2017;55:184–92. https://doi.org/10.1007/s11099-016-0219-5.
Vialet-Chabrand S, Matthews JSA, Simkin AJ, Raines CA, Lawson T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 2017;173:2163–79. https://doi.org/10.1104/pp.16.01767. PMID: 28184008.
Osei-Kwarteng M, Ayipio E, Moualeu-Ngangue D, Buck-Sorlin G, Stützel H. Interspecific variation in leaf traits, photosynthetic light response, and whole-plant productivity in amaranths (Amaranthus spp. L.). PLoS One. 2022;17(6):e0270674. https://doi.org/10.1371/journal.pone.0270674.
Zhong QL, Liu LB, Xu X, Yang Y, Guo YM, Xu HY, et al. Variations of plant functional traits and adaptive strategy of woody species in a karst forest of central Guizhou Province, southwestern China. Chi J Plant Ecol. 2018;42(5):562–72. https://doi.org/10.17521/cjpe.2017.0270.
Larsen KS, Andresen LC, Beier C, Jonasson S, Albert KR, Ambus P, et al. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: Synthesizing results of the CLIMAITE project after two years of treatments. Glob Chang Biol. 2011;17(5):1884–99. https://doi.org/10.1111/j.1365-2486.2010.02351.x.
Wright IJ, Dong N, Maire V, Prentice IC, Westoby M, Dıaz S, et al. Global climatic drivers of leaf size. Science. 2017;357:917–21. https://doi.org/10.1126/science.aal4760.
Malyshev AV, Arfin Khan MA, Beierkuhnlein C, Steinbauer MJ, Henry HAL, Jentsch A, et al. Plant responses to climatic extremes: within-species variation equals among-species variation. Glob Chang Biol. 2016;22(1):449–64. https://doi.org/10.1111/gcb.13114.
Pritzkow C, Williamson V, Szota C, Trouvé R, Arndt SK. Phenotypic plasticity and genetic adaptation of functional traits influences intra-specific variation in hydraulic efficiency and safety. Tree Physiol. 2020;40(2):215–29. https://doi.org/10.1093/treephys/tpz121.
Ahrens CW, Andrew ME, Mazanec RA, Ruthrof KX, Challis A, Hardy G, et al. Plant functional traits differ in adaptability and are predicted to be differentially affected by climate change. Ecol Evol. 2020;10:232–48. https://doi.org/10.1002/ece3.5890.
Wu ZY, Raven PH, Larsen K. Flora of China. Vol. 17. Verbenaceae through Solanaceae. Science Press, Beijing, and Missouri Botanical Garden Press. Nord J Bot. 1995;15:522.
Zhao YZ. On floristic geographical distribution of Caryopteris mongholica. Acta Scientiarum Naturalium Universitatis NeiMonggol. 1995;02:195–7.
Ji RX, Yu X, Ren TM, Chang Y, Li Z, Xia XX, et al. Genetic diversity and population structure of Caryopteris mongholica revealed by reduced representation sequencing. BMC Plant Biol. 2022;22(1):297. https://doi.org/10.1186/s12870-022-03681-y.
He YM, Wang C, Wang HT, Du ZY, Duan YZ. Effects of climate change on the potential suitable distribution area of Caryopteris mongolica. Acta Agrestia Sinice. 2023;31(02):540–50. https://doi.org/10.11733/j.issn.1007-0435.2023.02.028.
Yang S, Shi Z, Zhang M, Li Y, Gao J, Wang X, et al. Stoichiometry of carbon, nitrogen and phosphorus in shrub organs linked closely with Mycorrhizal strategy in Northern China. Front Plant Sci. 2021;12:687347. https://doi.org/10.3389/fpls.2021.687347.
Puglielli G, Carmona CP, Varone L, Laanisto L, Ricotta C. Phenotypic dissimilarity index: Correcting for intra- and interindividual variability when quantifying phenotypic variation. Ecology. 2022;103(11):e3806. https://doi.org/10.1002/ecy.3806.
Carmona CP, Pavanetto N, Puglielli G. funspace: an R package to build, analyze and plot functional trait spaces. 2023. https://doi.org/10.1101/2023.03.17.533069.
Salazar P, Navarro-Cerrillo R, Cruz G, Villar R. Intraspecific leaf functional trait variability of eight Prosopis pallida tree populations along a climatic gradient of the dry forests of northern Peru. J Arid Environ. 2018;152:12–20. https://doi.org/10.1016/j.jaridenv.2018.01.010.
Osnas JLD, Katabuchi M, Kitajima K, Wright SJ, Reich PB, Bael SAV, et al. Divergent drivers of leaf trait variation within species, among species, and among functional groups. Proc Natl Acad Sci USA. 2018;115(21):5480–5. https://doi.org/10.1073/pnas.1803989115.
Souza ML, Duarte AA, Lovato MB, Fagundes M, Valladares F, Lemos-Filho JP. Climatic factors shaping intraspecific leaf trait variation of a neotropical tree along a rainfall gradient. PLoS One. 2018;13(12):e0208512. https://doi.org/10.1371/journal.pone.0208512.
Liu LB, Yang J, Cao M, Song QH. Intraspecific trait variation of woody species reduced in a savanna community, southwest China. Plant Divers. 2021;44(2):163–9. https://doi.org/10.1016/j.pld.2021.06.002.
Xu F, Guo WH, Xu WH, Wei YH, Wang RQ. Leaf morphology correlates with water and light availability: What consequences for simple and compound leaves? Prog Nat Sci. 2009;19:1789–98. https://doi.org/10.1016/j.pnsc.2009.10.001.
Li YQ, Wang ZH. Leaf morphological traits: ecological function, geographic distribution and drivers. Chin J Plant Ecol. 2021;45(10):1154–72. https://doi.org/10.17521/cjpe.2020.0405.
Han WX, Fang JY, Guo DL, Zhang Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005;168(2):377–85. https://doi.org/10.1111/j.1469-8137.2005.01530.x.
Wang T, Yang YH, Ma WH. Storage, Patterns and environmental controls of soil phosphorus in china. Acta Scientiarum Naturalium Universitatis Pekinensis. 2008;6:945–52. https://doi.org/10.13209/j.0479-8023.2008.147.
Ma JJ, Ji CJ, Han M, Zhang TF, Yan XD, Hu D, et al. Comparative analyses of leaf anatomy of dicotyledonous species in Tibetan and Inner Mongolian grasslands. Sci China Life Sci. 2012;55:68–79. https://doi.org/10.1007/s11427-012-4268-0.
Wright IJ, Ackerly DD, Bongers F, Harms KE, IbarraManriquez G, Martine-Ramos M, et al. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Ann Bot-London. 2007;99:1003–15. https://doi.org/10.1093/aob/mcl066.
Yang YZ, Kang L, Zhao J, Qi N, Li RN, Wen ZM, et al. Quantifying leaf trait covariations and their relationships with plant adaptation strategies along an aridity gradient. Biology (Basel). 2021;10(10):1066. https://doi.org/10.3390/biology10101066.
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–7. https://doi.org/10.1038/nature02403.
Li YQ, Reich PB, Schmid B, Shrestha N, Feng X, Lyu T, et al. Leaf size of woody dicots predicts ecosystem primary productivity. Ecol Lett. 2020;23:1003–13. https://doi.org/10.1111/ele.13503.
Tsukaya H. Leaf shape: genetic controls and environmental factors. Int J Dev Biol. 2005;49(5–6):547–55. https://doi.org/10.1387/ijdb.041921ht.
Li ZH, Li ZF, Hong GY, Yang HF, Wang LJ, Gao XW. Prediction of potential distribution of caryopteris mongholica based on MaxEnt model in climate change context. Acta Bot Boreal -Occident Sin. 2022;42(07):1232–8. https://doi.org/10.7606/j.issn.1000-4025.2022.07.1232.
Ordonez JC, Van Bodegom PM, Witte JPM, Wright IJ, Reich PB, Aerts R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecol Biogeogr. 2009;18:137–49. https://doi.org/10.1111/j.1466-8238.2008.00441.x.
Hulshof CM, Swenson NG. Variation in leaf functional trait values within and across individuals and species: an example from a Costa Rican dry forest. Funct Ecol. 2010;24:217–23. https://doi.org/10.1111/j.1365-2435.2009.01614.x.
Baird AS, Taylor SH, Pasquet-Kok J, Vuong C, Sack L. Developmental and biophysical determinants of grass leaf size worldwide. Nature. 2021;592(7853):242–7. https://doi.org/10.1038/s41586-021-03370-0.
Traiser C, Klotz S, Uhl D, Mosbrugger V. Environmental signals from leaves—a physiognomic analysis of European vegetation. New Phytol. 2005;166:465–84. https://doi.org/10.1111/j.1469-8137.2005.01316.x.
Li FL, Bao WK. Elevational trends in leaf size of Campylotropis polyantha in the arid Minjiang River valley. SW China J Arid Environ. 2014;108:1–9. https://doi.org/10.1016/j.jaridenv.2014.04.011.
Acknowledgements
We would like to thank Meng Ji, Yuewen Yang, YuKun Xing at Inner Mongolia Academy of Forestry Science, for their help in plant identification.
Funding
This research was supported by the National Natural Science Foundation of China (31600484, 31770649).
Author information
Authors and Affiliations
Contributions
CL, XLX and WLY conceived and designed the research. CL, XY and RXJ contributed to the investigation. RXJ and MML processed the figures. XY analyzed data and wrote the original draft, and CL reviewed. All authors read and approved the manuscript. All authors agree to be accountable for the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We state that the methods used throughout the experiment were conducted in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Correlation coefficients of plant leaf functional traits in C. mongholica. *** means p<0.001, ** means p<0.01, * means p<0.05, means p<0.1.
Additional file 2: Fig. S2.
The effects of longitude, latitude, and altitude on the two principal component axes (T-PC1 and T-PC2) of the trait. Note: The solid line is significant and the dashed line is insignificant.
Additional file 3: Fig. S3.
Response curves between the probability of presence and climate variables of C. mongholica. The y-axis is the probability of existence of C. mongholica. Blue: mean±one standard deviation.
Additional file 4: Table S1.
Site characteristics for 40 sites of C. mongholica communities across its distribution in China.
Additional file 5: Table S2.
Bivariate relationships among climatic variables.
Additional file 6: Table S3.
Principal components analysis for climatic variables estimated for provenances (40 sites) of C. mongholica.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, X., Ji, R., Li, M. et al. Geographical variation in functional traits of leaves of Caryopteris mongholica and the role of climate. BMC Plant Biol 23, 394 (2023). https://doi.org/10.1186/s12870-023-04410-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04410-9