Abstract
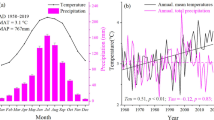
Minquartia guianensis Aubl. is a slow-growing species with several uses. In the juvenile state, it is well-adapted to low light conditions of the forest understory. However, it is still unknown how climate variability affects transpiration of this species, particularly under drought stress. In this study, we aimed to assess the effect of climatic variability on sap flow rates (SFR). SFR and radial growth were measured in six trees (14‒50 cm diameter) in 2015 and 2016. Climate (precipitation, irradiance, relative humidity and temperature) and soil water content (SWC) data were also collected. SFR tended to increase in the dry season, with a negative relationship between SFR and SWC and precipitation (p < 0.001), while there was a positive association between radial growth and monthly precipitation (p = 0.004). Irradiance and temperature were the environmental factors more closely correlated with SFR during daytime (p < 0.001), whereas relative humidity and vapor pressure deficit were the most important factors at night (p < 0.001). Although negative SFR were sometimes recorded at night, the mean nocturnal sap flow was positive and across trees the nighttime sap flow accounted for 12.5% of the total daily sap flow. Increased transpiration during the dry season suggests that the root system of Minquartia was able to extract water from deep soil layers. These results widen our understanding of the ecophysiology of Amazonian trees under drought and provide further insight into the potential effect of the forecasted decline in precipitation in the Amazon region.





Similar content being viewed by others
Abbreviations
- A S :
-
Active sapwood area
- D F :
-
Daily flow per tree
- log10 :
-
Base 10 logarithm
- MGR:
-
Monthly diameter growth rate
- PAR:
-
Photosynthetically active radiation
- RH:
-
Relative humidity
- RHmax :
-
Maximum RH
- RHmin :
-
Minimum RH
- SFR:
-
Sap flow rates
- SWC:
-
Soil water content
- T :
-
Temperature (°C)
- T max :
-
Maximum T
- T min :
-
Minimum T
- T mean :
-
Mean T
- VPD:
-
Vapor pressure deficit (atmospheric)
- V T :
-
Total sap flow per tree (volume of sap)
References
Barros FV, Bittencourt PRL, Brum M, Restrepo-Coupe N, Pereira L, Teodoro GS, Saleska SR, Borma LS, Christoffersen BO, Penha D, Alves LF, Lima AJN, Carneiro VMC, Gentine P, Lee J, Aragão LEOC, Ivanov V, Leal LSM, Araujo AC, Oliveira RS (2019) Hydraulic traits explain differential responses of Amazonian forests to the 2015 El Nino-induced drought. New Phytol 223(3):1253–1266
Bastable HG, Shuttleworth WJ, Dallarosa RLG, Fisch G, Nobre CA (1993) Observations of climate, albedo, and surface radiation over cleared and undisturbed Amazonian forest. Int J Climatol 13(7):783–796
Brinkmann WLF, Ribeiro MN, Pote JB (1971) Soil temperatures in the tertiary region of central Amazon. II. Cleared white sand areas. Acta Amazon 1:1–131
Brodersen CR, McElrone AJ (2013) Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Front Plant Sci 4:1–11
Burgess SSO, Adams MA, Turner NC, Beverly CR, Ong CK, Khan AAH, Bleby TM (2001) An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol 21(9):589–598
Burghardt M, Riederer M (2006) Cuticular transpiration. In: Riederer M, Müller C (eds) Biology of the plant cuticle. Blackwell Publishing, Oxford, pp 292–311
Camargo MAB, Marenco RA (2017) Tree growth over three years in response to monthly rainfall in central Amazonia. Dendrobiology 78(1):10–17
Costa ACL, Rowland L, Oliveira RS, Oliveira AAR, Binks OJ, Salmon Y, Vasconcelos SS, Junior JAS, Ferreira LV, Poyatos R, Mencuccini M, Meir P (2018) Stand dynamics modulate water cycling and mortality risk in droughted tropical forest. Glob Chang Biol 24(1):249–258
Dias DP, Marenco RA (2016) Tree growth, wood and bark water content of 28 Amazonian tree species in response to variations in rainfall and wood density. iForest 9(3):445–451
Domec JC, King JS, Noormets A, Treasure E, Gavazzi MJ, Sun G, McNulty SG (2010) Hydraulic redistribution of soil water by roots affects whole-stand evapotranspiration and net ecosystem carbon exchange. New Phytol 187(1):171–183
dos Santos VAHF, Ferreira MJ, Rodrigues JVFC, Garcia MN, Ceron JVB, Nelson BW, Saleska SR (2018) Causes of reduced leaf-level photosynthesis during strong El Niño drought in a Central Amazon forest. Glob Change Biol 24(9):4266–4279
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33(1):317–345
Fu R, Yin L, Li W, Arias PA, Dickinson RE, Huang L, Chakraborty S, Fernandes K, Liebmann B, Fisher R, Myneni RB (2013) Increased dry-season length over southern Amazonia in recent decades and its implication for future climate projection. Proc Natl Acad Sci USA 110(45):18110–18115
Gachet MS, Lecaro JS, Kaiser M, Brun R, Navarrete H, Muñoz RA, Bauer R, Schühly W (2010) Assessment of anti-protozoal activity of plants traditionally used in Ecuador in the treatment of leishmaniasis. J Ethnopharmacol 128:184–197
Goldsmith GR, Matzke NJ, Dawson TE (2013) The incidence and implications of clouds for cloud forest plant water relations. Ecol Lett 16(3):307–314
Goldstein G, Andrade JL, Meinzer FC, Holbrook NM, Cavelier J, Jackson P, Celis A (1998) Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant Cell Environ 21(4):397–406
Hasler N, Avissar R (2007) What controls evapotranspiration in the Amazon basin? J Hydrometeorol 8(3):380–395
Horna V, Schuldt B, Brix S, Leuschner C (2011) Environment and tree size controlling stem sap flux in a perhumid tropical forest of Central Sulawesi, Indonesia. Ann For Sci 68(5):1027–1038
Hunter JR (1991) Observations on the growth, ecology and uses of Minquartia guianensis, a humid tropical tree. Int Tree Crops J 6(4):221–238
IUCN-ARW (1998) Minquartia guianensis. The IUCN Red List of Threatened Species 1998: e.T32956A9737660. https://dx.doi.org/10.2305/IUCN.UK.1998.RLTS.T32956A9737660.en. Accessed on 05 March 20
Jones HG (1998) Stomatal control of photosynthesis and transpiration. J Exp Bot 49:387–398
Jones HG, Sutherland RA (1991) Stomatal control of xylem embolism. Plant Cell Environ 14(6):607–612
Juárez RIN, Hodnett MG, Fu R, Goulden ML, von Randow C (2007) Control of dry season evapotranspiration over the Amazonian forest as inferred from observations at a southern Amazon forest site. J Clim 20(12):2827–2839
Koch GW, Fredeen AL (2005) Transport challenges in tall trees. In: Holbrook NM, Zwieniecki MA (eds) Vascular transport in plants. Elsevier Academic Press, Amsterdam, pp 437–456
Kostaki KI, Coupel-Ledru A, Bonnell VC, Gustavsson M, Sun P, Mclaughlin FJ, Fraser DP, McLachlan DH, Hetherington AM, Dodd AD, Franklin KA (2020) Guard cells integrate light and temperature signals to control stomatal aperture. Plant Physiol 182(3):1404–1419
Kunert N (2016) Curios relationship revealed by looking at long term data sets: the geometry and allometric scaling of diel xylem sap flux in tropical trees. J Plant Physiol 205(1):80–83
Lee J-E, Frankenberg C, van der Tol C, Berry JA, Guanter L, Boyce CK, Fisher JB, Morrow E, Worden JR, Asefi S, Badgley G, Saatchi S (2013) Forest productivity and water stress in Amazonia: observations from GOSAT chlorophyll fluorescence. Proc Biol Sci 280(1761):20130171
Malhi Y, Wright J (2004) Spatial patterns and recent trends in the climate of tropical rainforest regions. Philos Trans R Soc Lond 359(1443):311–329
Malhi Y, Nobre AD, Grace J, Kruijt B, Pereira MGP, Culf A, Scott S (1998) Carbon dioxide transfer over a Central Amazonian rain forest. J Geophys Res 103(D24):31593–31612
Marenco RA, Vieira G (2005) Specific leaf area and photosynthetic parameters of tree species in the forest understorey as a function of the microsite light environment in central Amazonia. J Trop For Sci 17(2):265–278
Marenco RA, Antezana-Vera SA, Gouvêa PRS, Camargo MAB, Oliveira MF, Santos JKS (2014a) Physiology of Amazon tree species: photosynthesis, respiration and water relations. Rev Ceres 61(suppl):786–799
Marenco RA, Nascimento HC, Magalhães ND (2014b) Stomatal conductance in Amazonian tree saplings in response to variations in the physical environment. Photosynthetica 52(4):493–500
Marenco RA, Camargo MAB, Antezana-Vera SA, Oliveira MF (2017) Leaf trait plasticity in six forest tree species of central Amazonia. Photosynthetica 55(4):679–688
Marles RJ, Farnsworth NR, Neill DA (1989) Isolation of a novel cytotoxic polyacetylene from a traditional anthelmintic medicinal plant Minquartia guianensis. J Nat Prod 52(2):261–266
Motzer T, Munz N, Kuppers M, Schmitt D, Anhuf D (2005) Stomatal conductance, transpiration and sap flow of tropical montane rain forest trees in the southern Ecuadorian Andes. Tree Physiol 25(10):1283–1293
O’Brien JJ, Oberbauer SF, Clark DB (2004) Whole tree xylem sap flow responses to multiple environmental variables in a wet tropical forest. Plant, Cell Environ 27(5):551–567
Oliveira MF, Marenco RA (2019) Photosynthesis and biomass accumulation in Carapa surinamensis (Meliaceae) in response to water stress at ambient and elevated CO2. Photosynthetica 57(1):137–146
Ranzani G (1980) Identificação e caracterização de alguns solos da Estação Experimental de Silvicultura Tropical do INPA [Identificationandcharacterizationof some soilsoftheINPA Tropical Forestry Experimental Station]. Acta Amazon 10(1):7–41
Restrepo-Coupe N, Rocha HR, Hutyra LR, da Araujo AC, Borma LS, Christoffersen B, Cabral OMR, Camargo PB, Cardoso FL, Costa ACL, Fitzjarrald DR, Goulden ML, Kruijt B, Maia JMF, Malhi YS, Manzii AO, Miller SD, Nobre AD, von Randow C, Sá LDA, Sakai RK, Tota J, Wofsy SC, Zanchi FB, Saleska SR (2013) What drives the seasonality of photosynthesis across the Amazon basin? A cross-site analysis of eddy flux tower measurements from the Brasil flux network. Agric For Meteorol 182:128–144
Ruiz L, Ruiz L, Maco M, Cobos M, Gutierrez-Choquevilca AL, Roumy V (2011) Plants used by native Amazonian groups from the Nanay River (Peru) for the treatment of malaria. J Ethnopharmacol 133(2):917–921
Rundel PW (1982) Water uptake by organs other than roots. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Water relations and carbon assimilation. Springer, Berlin, pp 111–134
Ryan MG, Hubbard RM, Clark DA, Sanford RL (1994) Woody-tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits. Oecologia 100(3):213–220
Saatchi SS, Houghton RA, Alvalá RCS, Soares JV, Yu Y (2007) Distribution of aboveground live biomass in the Amazon basin. Glob Change Biol 13(4):816–837
Salati E (1987) The forest and the hydrological cycle. In: Dickinson RE (ed) The geophysiology of Amazonia: vegetation and climate interactions. Wiley, New York, pp 273–296
Saleska SR, Didan K, Huete AR, Da Rocha HR (2007) Amazon forests green-up during 2005 drought. Science 318(5850):612
Schulze E-D, Čermák J, Matyssek M, Penka M, Zimmermann R, Vasícek F, Gries W, Kučera J (1985) Canopy transpiration and water fluxes in the xylem of the trunk of Larix and Picea trees- a comparison of xylem flow, porometer and cuvette measurements. Oecologia 66(4):475–483
Taylor AM, Gartner BL, Morrell JJ (2002) Heartwood formation and natural durability-a review. Wood Fiber Sci 34(4):587–611
Tyree MT, Zimmermann MH (2002) The cohesion-tension theory of sap ascent–xylem dysfunction. In: Tyree MT, Zimmermann MH (eds) Xylem structure and the ascent of sap. Springer, Berlin, pp 49–132
Vandegehuchte MW, Steppe K (2012) Improving sap flux density measurements by correctly determining thermal diffusivity, differentiating between bound and unbound water. Tree Physiol 32:930–942
Zhao CY, Si JH, Feng Q, Yu TF, Li PD (2017) Comparative study of daytime and nighttime sap flow of Populus euphratica. Plant Growth Regul 82(2):353–362
Zhao H, Yang S, Guo X, Peng C, Gu X, Deng C, Chen L (2018) Anatomical explanations for acute depressions in radial pattern of axial sap flow in two diffuse-porous mangrove species: implications for water use. Tree Physiol 38(2):276–286
Zhao C, Si J, Feng Q, Yu T, Li P, Forster MA (2019) Nighttime transpiration of Populus euphratica during different phenophases. J For Res 30(2):435–444
Acknowledgements
Thanks to the Ministério da Ciência, Tecnologia e Inovações (MCTI-INPA, PRJ-15.120), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, fellowship to RAM–303907/2018-5, and scholarship to SAAV), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, code 001) and Fundação de Amparo a Pesquisa do Amazonas (FAPEAM) for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study is supported by research project (MCTI/INPA: PRJ-15.120).
The online version is available at http://www.springerlink.com.
Corresponding editor: Zhu Hong.
Rights and permissions
About this article
Cite this article
Antezana-Vera, S.A., Marenco, R.A. Sap flow rates of Minquartia guianensis in central Amazonia during the prolonged dry season of 2015–2016. J. For. Res. 32, 2067–2076 (2021). https://doi.org/10.1007/s11676-020-01193-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01193-9