Abstract
The Himalayas are characterized by a broad gradient of bioclimatic zones along their elevation. However, less is known how forest growth responds to climatic change along elevation. In this study, four standard tree-ring width chronologies of Himalayan fir (Abies spectabilis) were developed, spanning 142–649 years along an elevation gradient of 3076–3900 m a.s.l. Principal component analysis classified the four chronologies into two groups; the ones at lower elevations (M1 and M2) and higher elevations (M3 and M4) show two distinct growth trends. Radial growth is limited by summer (June–August) precipitation at M3, and by precipitation during spring (March–May) and summer at M4. It is limited by spring temperatures and winter precipitation (December–February) at M1. Tree-ring width chronologies also significantly correlate with winter and spring Palmer Drought Severity Index (PDSI) at M1, and with summer PDSI at M3 and M4. Thus, Himalayan fir growth at high elevations is mainly limited by moisture stress rather than by low temperatures. Furthermore, the occurrence of missing rings coincides with dry periods, providing additional evidence for moisture limitation of Himalayan fir growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, climate change has significantly affected the composition, structure, and dynamics of forest ecosystems (IPCC 2014; Allen et al. 2015). These effects are more pronounced on tree physiology and population dynamics in mountainous regions (Körner 2012). However, forest growth at high altitudes is often subjected to environmental gradients associated with elevation (LaMarche 1974; Hughes and Funkhouser 2003). As showed by different dendroclimatological studies, tree growth is limited by moisture at lower elevations (Fritts et al. 1965; Leel et al. 2007), and by temperatures at higher elevations (Savva et al. 2006; Fan et al. 2008). However, some studies have shown uniform growth response along elevation gradients (Liu et al. 2006; Liang et al. 2010; Wang et al. 2015). Therefore, a better understanding of tree growth response to climate along elevation gradients is highly important to assess the impacts of climate change on forest ecosystems.
The Himalayas are characterized by a broad gradient of bioclimatic zones and diverse forests along elevation gradients. It is one of the world's most vulnerable regions to global warming, with a warming rate higher than the global average (Shrestha et al. 2012). Warming-induced drought stress has caused a higher occurrence of missing annual rings of high-elevation forests in the central Himalayas (Liang et al. 2014), and declining recruitment at the world’s highest juniper shrublines in recent decades on the Tibetan Plateau (Lu et al. 2019). Thus, ongoing warming is expected to further alter drought-sensitive forest ecosystems along elevations. To date, few studies have been conducted along the elevation gradients in the Himalayas (Kharal et al. 2017; Sohar et al. 2017).
In the central Himalayas, several studies have investigated tree growth response to climate in recent decades (Cook et al. 2003; Dawadi et al. 2013; Liang et al. 2014, 2019; Thapa et al. 2014; Panthi et al. 2017; Shrestha et al. 2017; Sigdel et al. 2018a). Based on published results, tree growth showed diverse responses to climatic change in the central Himalayas. Some studies have indicated that growth was primarily controlled by variations in temperature (Cook et al. 2003; Gaire et al. 2014; Thapa et al. 2014; Kharal et al. 2017), while other studies have shown that tree growth was significantly controlled by precipitation at higher elevations (Dawadi et al. 2013; Liang et al. 2014, 2019; Panthi et al. 2017). Meanwhile, both temperature and precipitation can have a significant influence on tree growth (Sano et al. 2005; Sohar et al. 2017). To better understand different growth responses to climate change in the central Himalayas, it is necessary to investigate growth responses along elevation gradients.
The objective of this study is to examine how Himalayan fir (Abies spectabilis (D. Don) Mirb.) growth responds to climate along an elevation gradient in the Manang valley of the central Himalayas (Nepal). Himalayan fir is an ecologically important species of sub-alpine forest ecosystems which form natural treelines in the central Himalayas (Sigdel et al. 2018b). Given that precipitation in the central Himalayas decreases with increasing elevations above 3000 m (Liang et al. 2014), it was hypothesized that moisture stress rather than low temperatures primarily control the growth of Himalayan fir at high elevations.
Materials and methods
Study area
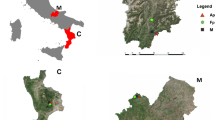
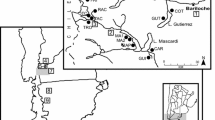
The sampling sites are located along an elevation gradient of the Manang valley (83°40′–84°40′E and 28°20′–29°0′N). The elevation of the sampled Himalayan fir forest ranges from 3076 to 3900 m a.s.l. (Fig. 1).
Climate in the central Himalayas is influenced by two different weather circulation systems (Indian monsoons in the summer, and westerly jet streams in the winter), with a high inter-annual variability (Yao et al. 2012). The upper part of the Manang valley receives much less annual precipitation as it is surrounded by the Annapurna Mountain massif. Monthly mean maximum and minimum temperatures at Chame climate station (28°33′N, 84°14′E; 2680 m) are 11.3 °C and − 1.6 °C in winter, and 20.6 °C and 9.6 °C in summer (Fig. 2). Annual precipitation at Manangbhot climate station (28°40′N, 84°01′E; 3520 m) is about 400 mm (1977–2013) and ca. 65% of total annual precipitation occurs during the monsoon season. According to the climate data, annual maximum temperatures have been increasing significantly, and total annual precipitation has been significantly decreasing (Fig. 3).
Sample collection and dendrochronological analyses
Increment cores were extracted from a natural Himalayan fir stand using an increment borer. Core samples were collected along an elevation gradient from 3076 to 3900 m at every 250–300 m differences. The sites cover the lowest elevation limit and alpine timberline of Himalayan fir in the Manang valley. At each sampling site, 15–30 mature, healthy trees were selected and one to two cores were collected from each tree at breast height. A total of 33, 48, 30 and 25 increment cores were collected and used to develop chronologies from the sampling sites M1 (3076 m), M2 (3335 m), M3 (3666 m) and M4 (3900 m), respectively (Table 1).
Samples were stored in paper tubes and labeled. Preparation and processing were carried out following standard dendrochronological methods (Cook and Kairiukstis 2013). The dried cores were fixed in wooden slots and core surfaces smoothed with consecutively finer grades of sand papers until ring boundaries were clearly visible. Tree-ring widths were measured using a LINTAB measuring system at 0.01 mm precision (Rinntech, Heidelberg, Germany). The measurement and cross-dated series were further verified using the COFECHA software (Holmes 1983). Standardization of cross-dated tree-ring width data was performed using the computer program ARSTAN (Cook 1985). To remove any biological trends and maximize the strength of climatic signals of the chronology, all raw series were detrended and standardized by negative exponential curves or a 67-year cubic smoothing spline. Standard chronologies were used for all analyses.
Mean inter-series correlations (RBAR) and expressed population signals (EPS) were calculated with a 50-year moving window with a 25-year overlap (Wigley et al. 1984). The common period (1930–2013) with an EPS ≥ 0.85 was used for further analysis except for the chronology at M1 (EPS = 0.78). A lower EPS value at M1 may be due to moderate climatic conditions or low sample depth.
Climate-growth relationships
Tree growth response to climate was investigated by calculating Pearson’s correlation between the four standard chronologies and seasonal (winter ‘DJF’, spring ‘MAM’ and summer ‘JJA’), as well as monthly climate data from 1979 to 2013 retrieved from high resolution (30 arc sec, ~ 1 km) satellite data CHELSA (Climatologies at High resolution for the Earth’s Land Surface Areas) (Karger et al. 2017). Due to a lack of climatic data along sampling gradients, satellite data CHELSA was used to analyze climate-tree growth relationships. These climatic data showed high correlations with temperature records of Chame climate station (2680 m) from 1979 to 2012, and with precipitation records from 1979 to 2013 at Manangbhot climate station (3420 m). For monthly mean maximum and minimum temperatures, the correlation coefficient (r) ranged from 0.89 to 0.90 (p < 0.001). For precipitation data (r) ranged from 0.43 to 0.44 (p < 0.001). This demonstrates that the CHELSA climate data represents the climatic conditions of the four sampling sites.
Principal component analysis (PCA) was carried out using the four chronologies during the common period 1930–2013. PCA was calculated on the covariance matrix of the chronologies and only two principal components (PC1 and PC2) were retained as they had eigenvalues greater than one. Monthly and seasonal means of climatic variables were correlated with PC1 and PC2 to investigate the extent of common growth variations through time. Linear association between the two factors was calculated by employing partial correlation after excluding the third factor.
Results
Statistics of the chronologies
The longest chronology (649 years) was developed from the lowest elevation (M1) data, followed by a 402-year chronology from the mid-elevation (M2), a 229-year chronology from the higher-mid elevation (M3), and a 142-year chronology from the highest site (M4) (Table 1; Fig. 4). Of the total rings measured from the four sampling sites, locally missing rings accounted for 0.16%. During the past 200 years, locally missing rings were observed in 1820, 1821, 1848, 1849, 1864, 1866, 1867, 1898, 1918, 1968, 1971, 1978, 1979, 2000, 2002, 2003, 2004, 2010 and 2011. Frequency of locally missing rings increased with elevation, except for the M4 site where there were no missing rings.
Within the chronologies, RBAR ranged from 0.15 to 0.61, and EPS crossed 0.85 from 1930 to 2013 at all sites except M1 (Table 1). Mean sensitivity (MS) ranged from 0.15 to 0.26. Principal component analysis showed that PC1 and PC2 explained 41.7% and 32.6% of variance, respectively (Fig. 5). All chronologies did not load positively on PC1. The higher elevation sites (M3 and M4) had positive loadings on PC1, while the lower elevation sites (M1 and M2) demonstrated positive loadings on PC2. (Fig. 5). Significant correlations have been observed between adjacent sites M1 and M2 (r = 0.30, p < 0.01), and M3 and M4 (r = 0.66, p < 0.001), while there were no significant correlations between the chronologies at lower and higher elevations (Table 2).
Loadings of the first and second principal components (PC1 and PC2) calculated on the covariance matrix of the four standard tree-ring width chronologies (Fig. 4) and considering a common period (1930–2013 AD). Numbers indicate site elevation
Climate-growth relationships
The growth of Himalayan fir varied in response to climatic change along the elevation gradient. Tree-ring width chronologies at M4 correlated positively with precipitation during spring (r = 0.43, p < 0.05), May (r = 0.46, p < 0.01), and summer (r = 0.69, p < 0.001) (Fig. 6). Tree growth at M3 also showed positive correlation with summer precipitation (r = 0. 59, p < 0.001). On the other hand, tree growth at both M3 and M4 was weakly associated with temperature. Furthermore, growth of Himalayan fir at the M1 site was positively correlated with winter precipitation (r = 0.52, p < 0.01) and negatively with spring temperatures (r = − 0.54, p < 0.01). In particular, the M1 chronology showed a negative correlation with April temperatures (r = − 0.55, p < 0.01). Lastly, M2 chronologies were significantly positive correlated with July temperatures (r = 0.42, p < 0.05) (Fig. 6). The first-order difference series for the chronologies and climatic data also supported this analysis (Fig. 6).
Pearson correlation coefficients of ring-width indices and average monthly and seasonal temperatures, and total monthly and seasonal precipitation, including the previous year October (pO) to the present-year September (S), based on climate data from CHELSA. DJF: December to February; MAM: March to May; JJA: June to August, including seasonal first-order different series
Tree growth showed positive significant correlation with Palmer Drought Severity Index (PDSI) during spring (r = 0.38, p < 0.05) and summer (r = 0.58, p < 0.001) at M3, and during summer at M4 (r = 0.34, p < 0.05). At M1, tree growth correlated positively with PDSI during winter (r = 0.42, p < 0.05) and spring (r = 0.38, p < 0.05) (Fig. 6).
Partial correlation analysis showed significant correlations between summer precipitation and M3 (r = 0.59, p < 0.001), and M4 chronologies (r = 0.66, p < 0.001) when controlling for the effect of summer temperatures. In addition, M4 chronology and spring precipitation correlated significantly (r = 0.43, p < 0.05) when reflecting the influence of spring temperatures. In addition, there was significant correlation between M1 chronology and winter precipitation (r = 0.47, p < 0.01), and spring temperatures (r = − 0.55, p < 0.001) after excluding the effect of winter temperatures and spring precipitation.
PC1 showed significant and positive correlation with summer precipitation (r = 0.48, p < 0.01), while weak correlation with summer temperature (r = 0.179, p = 0.305) (Fig. 7). It also had significant positive correlation with annual precipitation from July of the previous year to June of the current year (r = 0.35, p < 0.05). However, PC2 was significantly negative correlated with spring temperatures (r = − 0.52, p < 0.01) (Fig. 7).
Discussion
Locally missing rings and drought
The occurrence of missing rings suggests that widespread drought influenced tree growth in the Manang valley. The missing rings synchronized with large-scale drought events in Nepal during 1967–1973 and 1999–2001 (Sigdel and Ikeda 2010). Most of the locally missing rings in our study area also correspond with dry periods (1848–1849, 1859–1876, 1887–1901, and 1964–1973) in the western Himalayas (Ram 2012). In addition, some of the missing-ring years coincided with those for Himalayan birch (Betula utilis D. Don) in 1968, 2000, 2003 and 2004 (Liang et al. 2014). Furthermore, the occurrence of narrow rings in the Himalayan fir chronologies also coincide with the drought periods (1807–1828, 1892–1898, 1915–1927) in the central Himalayas (Panthi et al. 2017), suggesting that frequency of missing rings may archive the signals of past drought events. However, the absence of missing rings at the uppermost sampling site might be due to the presence of young trees with good vigor. Hence, frequent locally missing rings provide an additional insight about extreme climatic conditions and their effect on tree growth in the semi-arid regions of the central Himalayas.
Climate-growth relationships
Relationships between the radial growth of Himalayan fir and climate were not uniform along the elevation gradient. Variations in aspect, slope, and elevation of sites may influence tree growth (Lyu et al. 2017), hence obscuring climatic signals embedded in tree rings. In this study, the growth of Himalayan fir at high altitudes was mainly constrained by moisture stress rather than by growing season temperatures. Stronger precipitation signals at higher elevations further support the premise that precipitation decreases with increasing elevation in the central Himalayas (Liang et al. 2014). Moisture stress during pre-monsoon and monsoon seasons seems to limit Himalayan fir growth across the elevation gradient in our study area. Radial growth has a negative correlation with pre-monsoon temperatures and a positive correlation with PDSI at the M1 site, suggesting moisture stress on tree growth. The negative correlation between PC2 and spring temperatures further highlight the importance of moisture availability on tree growth. Such results have been reported for different tree species in the Himalayas and on the Tibetan Plateau (Yadav et al. 2004; Sano et al. 2005; Borgaonkar et al. 2011; Dawadi et al. 2013; Liang et al. 2014; Kharal et al. 2017; Panthi et al. 2017; Mou et al. 2019; Sigdel et al. 2018a). Although warming temperatures enhance xylem cell production, the thermal threshold is an important indicator of tree growth at the alpine treeline (Li et al. 2017; Liang and Camarero 2018). High spring temperatures can cause water deficits by enhancing evapotranspiration and hence delay the onset of xylogenesis or wood formation (Ren et al. 2018). Increased temperatures coupled with lower precipitation in the early growing season can cause drought stress, stomata closure and reduced carbon assimilation (Wang et al. 2005). Likewise, significant positive correlations between tree growth at the M1 site and total winter precipitation might be related to the replenishing of soil moisture before the onset of xylogenesis. Further, tree growth at the M2 site was only significantly correlated with July temperatures. M1 and M2 chronologies are significantly correlated with each other (Table 2). However, it is difficult to understand why tree growth at the M2 site did not show signals of drought stress. Future studies should investigate the causes for different growth responses to climate at the M2 site. Likewise, site-specific climate data could better explain such climate-growth relationships.
Generally, temperature is a growth limiting factor for trees at higher elevations (Liu et al. 2005; Fan et al. 2008; Affolter et al. 2010; Bayramzadeh et al. 2018; Yu and Liu 2018). In the central Himalayas, spring precipitation is considered to be a driving factor for tree growth, in particular in high-elevation forests (Dawadi et al. 2013; Liang et al. 2014, 2019; Panthi et al. 2017; Tiwari et al. 2017), as well as in subtropical forests (Sigdel et al. 2018a). Not only tree growth, treeline upward shift across the central Himalayas was also primarily determined by spring precipitation (Sigdel et al. 2018b). However, this study shows that tree growth at high elevations is limited by precipitation during spring and summer seasons, possibly due to much drier climate conditions at higher elevations of the Manang valley compared with other research areas (Bhattarai et al. 2004). Positive correlations between tree growth and summer PDSI (Palmer Drought Severity Index) further suggests that moisture shortages limit tree growth. On the other hand, precipitation and surface water during summers are the major source of moisture for tree growth in arid locations (Littell et al. 2008), supporting our main findings. Similar cases of growth-climate relationships have been reported for the cold and arid regions of the northeastern Tibetan Plateau (Chen et al. 2011; Yang et al. 2013; Lyu et al. 2019).
Rising temperatures and decreasing precipitation could further accelerate drought stress in the future, especially in the highly sensitive, semi-arid trans-Himalayan region by increasing evapotranspiration and vapor pressure deficits (Wang et al. 2013; Ding et al. 2018). In addition, competition between nearby trees for soil moisture may further exacerbate moisture stress for tree growth (Liang et al. 2016). Warming and drying climates have already caused growth decline, canopy dieback, and even mortality for drought-prone forests on the Tibetan Plateau and in the Himalayas (Liang et al. 2014, 2016; Fang et al. 2018).
Conclusions
The growth of Himalayan fir in the central Himalayas is primarily limited by moisture stress. Despite different climatic signals along the elevation gradient, moisture stress is a common force for growth variations. Under climate warming in recent decades, the growth of Himalayan fir in the Manang valley will undergo increasing moisture stress in the future. These findings contribute to a better understanding of the impacts of climate change on forest ecosystems in the central Himalayas.
References
Affolter P, Büntgen U, Esper J, Rigling A, Weber P, Luterbacher J, Frank D (2010) Inner alpine conifer response to 20th century drought swings. Eur J For Res 129(3):289–298
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the anthropocene. Ecosphere 6(8):1–55
Bayramzadeh V, Zhu H, Lu X, Attarod P, Zhang H, Li X, Asad F, Liang E (2018) Temperature variability in northern Iran during the past 700 years. Sci Bull 63:462–464
Bhattarai KR, Vetaas OR, Grytnes JA (2004) Relationship between plant species richness and biomass in an arid sub-alpine grassland of the central Himalayas, Nepal. Folia Geobot 39:57–71
Borgaonkar HP, Sikder AB, Ram S (2011) High altitude forest sensitivity to the recent warming: a tree-ring analysis of conifers from western Himalaya, India. Quat Int 236(1–2):158–166
Chen F, Yuan Y, Wei W (2011) Climatic response of Picea crassifolia tree-ring parameters and precipitation. J Arid Environ 75:1121–1128
Cook ER (1985) A time series approach to tree ring standardization [dissertation]. University of Arizona, Tuscon, p 171
Cook ER, Kairiukstis LA (2013) Methods of dendrochronology: applications in the environmental sciences. Springer, Berlin, p 394
Cook ER, Krusic PJ, Jones PD (2003) Dendroclimatic signals in long tree-ring chronologies from the Himalayas of Nepal. Int J Clim 23(7):707–732
Dawadi B, Liang E, Tian L, Devkota LP, Yao T (2013) Pre-monsoon precipitation signal in tree rings of timberline Betula utilis in the central Himalayas. Quat Int 283:72–77
Ding JZ, Yang T, Zhao YT, Liu D, Wang XY, Yao YT, Peng SS, Wang T, Piao SL (2018) Increasingly important role of atmospheric aridity on Tibetan alpine grasslands. Geophys Res Lett 45(6):2852–2859
Fan ZX, Bräuning A, Cao KF (2008) Annual temperature reconstruction in the central Hengduan Mountains, China, as deduced from tree rings. Dendrochronologia 26(2):97–107
Fang O, Alfaro RI, Zhang QB (2018) Tree rings reveal a major episode of forest mortality in the late 18th century on the Tibetan Plateau. Glob Planet Change 163:44–50
Fritts HC, Smith DG, Cardis JW, Budelsky CA (1965) Tree-ring characteristics along a vegetation gradient in northern Arizona. Ecology 46(4):393–401
Gaire NP, Koirala M, Bhuju DR, Borgaonkar HP (2014) Treeline dynamics with climate change at the central Nepal Himalaya. Clim Past 10(4):1277–1290
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78
Hughes MK, Funkhouser G (2003) Frequency-dependent climate signal in upper and lower forest border tree rings in the mountains of the Great Basin. Clim Change 59:233–244
IPCC (2014) Climate Change 2014-impacts, adaptation and vulnerability: regional aspects. Cambridge University Press, Cambridge, p 688
Karger DN, Conrad O, Bohner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2017) Climatologies at high resolution for the earth's land surface areas. Sci Data 4, 170122
Kharal DK, Thapa UK, St. George S, Meilby H, Rayamajhi S, Bhuju DR (2017) Tree-climate relations along an elevational transect in Manang Valley, central Nepal. Dendrochronologia 41:57–64
Körner C (2012) Alpine treelines: functional ecology of the global high elevation tree limits. Springer, Basel, p 220
LaMarche VC (1974) Frequency-dependent relationships between tree-ring series along an ecological gradient and some dendroclimatic implications. Tree-Ring Bull 34:1–20
Leel S, Melvin TM, Grabner M, Wimmer R, Briffa KR (2007) Tree-ring growth variability in the Austrian Alps: the influence of site, altitude, tree species and climate. Boreas 36(4):426–440
Li X, Liang E, Gričar J, Rossi S, Čufar K, Ellison AM (2017) Critical minimum temperature limits xylogenesis and maintains treelines on the southeastern Tibetan Plateau. Sci Bull 62(11):804–812
Liang E, Camarero JJ (2018) Threshold-dependent and non-linear associations between temperature and tree growth at and below the alpine treeline. Trees 32(2):661–662
Liang E, Wang Y, Xu Y, Liu B, Shao X (2010) Growth variation in Abies georgei var. smithii along altitudinal gradients in the Sygera Mountains, southeastern Tibetan Plateau. Trees 24(2):363–373
Liang E, Dawadi B, Pederson N, Eckstein D (2014) Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 95(9):2453–2465
Liang E, Leuschner C, Dulamsuren C, Wagner B, Hauck M (2016) Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Clim Change 134(1–2):163–176
Liang E, Dawadi B, Pederson N, Piao S, Zhu H, Sigdel SR, Chen D (2019) Strong link between large tropical volcanic eruptions and severe droughts prior to monsoon in the central Himalayas revealed by tree-ring records. Sci Bull. https://doi.org/10.1016/j.scib.2019.05.002
Littell JS, Peterson DL, Tjoelker M (2008) Douglas-fir growth in mountain ecosystems: water limits tree growth from stand to region. Ecol Monogr 78(3):349–368
Liu X, Qin D, Shao X, Chen T, Ren J (2005) Temperature variations recovered from tree-rings in the middle Qilian Mountain over the last millennium. Sci China Ser D Earth Sci 48(4):521–529
Liu LS, Shao XM, Liang EY (2006) Climate signals from tree ring chronologies of the upper and lower treelines in the Dulan region of the northeastern Qinghai-Tibetan Plateau. J Integr Plant Biol 48(3):278–285
Lu X, Liang E, Wang Y, Babast F, Leavitt SW, Camarero JJ (2019) Past the climate optimum: Recruitment is declining at the world's highest juniper shrublines on the Tibetan Plateau. Ecology 100(2):e02557
Lyu L, Suvanto S, Nöjd P, Henttonen HM, Mäkinen H, Zhang QB (2017) Tree growth and its climate signal along latitudinal and altitudinal gradients: Comparison of tree rings between Finland and the Tibetan Plateau. Biogeosciences 14:3083–3095
Lyu L, Zhang QB, Pellatt MG, Büntgen U, Li MH, Cherubini P (2019) Drought limitation on tree growth at the Northern Hemisphere’s highest tree line. Dendrochronologia 53:40–47
Mou YM, Fang O, Cheng X, Qiu H (2019) Recent tree growth decline unprecedented over the last four centuries in a Tibetan juniper forest. J For Res 30(4):1429–1436
Panthi S, Bräuning A, Zhou ZK, Fan ZX (2017) Tree rings reveal recent intensified spring drought in the central Himalaya, Nepal. Glob Planet Change 157:26–34
Ram S (2012) Tree growth-climate relationships of conifer trees and reconstruction of summer season Palmer Drought Severity Index (PDSI) at Pahalgam in Srinagar, India. Quat Int 254:152–158
Ren P, Rossi S, Camarero JJ, Ellison AM, Liang E, Penuelas J (2018) Critical temperature and precipitation thresholds for the onset of xylogenesis of Juniperus przewalskii in a semi-arid area of the north-eastern Tibetan Plateau. Ann Bot 121(4):617–624
Sano M, Furuta F, Kobayashi O, Sweda T (2005) Temperature variations since the mid-18th century for western Nepal, as reconstructed from tree-ring width and density of Abies spectabilis. Dendrochronologia 23(2):83–92
Savva Y, Oleksyn J, Reich PB, Tjoelker MG, Vaganov EA, Modrzynski J (2006) Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees 20(6):735–746
Shrestha UB, Gautam S, Bawa KS (2012) Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS ONE 7(5):e36741
Shrestha KB, Chhetri PK, Bista R (2017) Growth responses of Abies spectabilis to climate variations along an elevational gradient in Langtang National Park in the central Himalaya, Nepal. J For Res 22(5):274–281
Sigdel M, Ikeda M (2010) Spatial and temporal analysis of drought in Nepal using standardized precipitation index and its relationship with climate indices. J Hydrol Meteorol 7(1):59–74
Sigdel SR, Dawadi B, Camarero J, Liang E, Leavitt S (2018a) Moisture-Limited Tree Growth for a Subtropical Himalayan Conifer Forest in Western Nepal. Forests 9(6):340
Sigdel SR, Wang Y, Julio Camarero J, Zhu H, Liang E, Penuelas J (2018b) Moisture-mediated responsiveness of treeline shifts to global warming in the Himalayas. Glob Change Biol 24(11):5549–5559
Sohar K, Altman J, Lehečková E, Doležal J (2017) Growth–climate relationships of Himalayan conifers along elevational and latitudinal gradients. Int J Clim 37(5):2593–2605
Thapa UK, Shah SK, Gaire NP, Bhuju DR (2014) Spring temperatures in the far-western Nepal Himalaya since AD 1640 reconstructed from Picea smithiana tree-ring widths. Clim Dyn 45(7–8):2069–2081
Tiwari A, Fan ZX, Jump AS, Li SF, Zhou ZK (2017) Gradual expansion of moisture sensitive Abies spectabilis forest in the Trans-Himalayan zone of central Nepal associated with climate change. Dendrochronologia 41:34–43
Wang T, Ren H, Ma K (2005) Climatic signals in tree ring of Picea schrenkiana along an altitudinal gradient in the central Tianshan Mountains, northwestern China. Trees 19(6):736–742
Wang SY, Yoon JH, Gillies RR, Cho C (2013) What caused the winter drought in western Nepal during recent years? J Clim 26(21):8241–8256
Wang Z, Yang B, Deslauriers A, Bräuning A (2015) Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in northwestern China. Trees 29(1):25–34
Wigley TML, Briffa KR, Jones PD (1984) On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J Clim Appl Meteorol 23(2):201–213
Yadav RR, Park WK, Singh J, Dubey B (2004) Do the western Himalayas defy global warming? Geophys Res Lett 31:L17201
Yang B, He M, Melvin TM, Zhao Y, Briffa KR (2013) Climate control on tree growth at the upper and lower treelines: a case study in the Qilian Mountains. Tibetan Plateau. PLoS One 8(7):e69065
Yao T, Thompson L, Yang W, Yu W, Gao Y, Guo X, Yang X, Duan K, Zhao H, Xu B, Pu J, Lu A, Xiang Y, Kattel DB, Joswiak D (2012) Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat Clim Change 2(9):663–667
Yu J, Liu QJ (2018) Larix olgensis growth–climate response between lower and upper elevation limits: an intensive study along the eastern slope of the Changbai Mountains, northeastern China. J For Res. https://doi.org/10.1007/s11676-018-0788-1
Acknowledgements
We thank the Kathmandu Center for Research and Education, CAS-TU, for help during the fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study is supported by National Natural Science Foundation of China (41661144040), Chinese Academy of Sciences President’s International Fellowship Initiative (2018PC0040).
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rai, S., Dawadi, B., Wang, Y. et al. Growth response of Abies spectabilis to climate along an elevation gradient of the Manang valley in the central Himalayas. J. For. Res. 31, 2245–2254 (2020). https://doi.org/10.1007/s11676-019-01011-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-01011-x