Abstract
In the current work, a typical NiCrAlY alloy and two novel amorphous Fe-based alloys were arc-sprayed for the desired application as insulative coatings in cryogenic environments such as for the inner walls of LH2 tanks. For the experiments, nitrogen was used as process gas, while the stand-off distance and number of passes were varied. The deposition efficiency, tensile adhesive strength, hardness, durability under cryogenic conditions, and thermal diffusivity were investigated. Another focus included the visual and chemical characterization of the microstructures and potential changes in these due to the harsh temperature regime. Additionally, the residual stresses within the coatings were measured by the incremental hole drilling method based on electronic speckle pattern interferometry and correlated with the other coating properties. This was done to estimate the influence of the stress state on possible coating delamination in the tanks over a longer period. The results demonstrate coatings with low, but varying porosity and oxide content as well as material-dependent correlations to electrical conductivity. In particular, the amorphous Fe-based coatings reveal homogeneous coating structures and promising properties in terms of insulation capacity for use in various cryogenic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermally sprayed coatings or composite coatings can serve as a key technology for the energy turnaround in various new industrial applications. For instance, currently, there is little experience in maritime production, offshore storage, and transport of large quantities of liquid hydrogen (LH2) due to the developing market (Ref 1). Instead, current tank designs refer to standard onshore storage and transport applications with vacuum-insulated double wall hulls made from austenitic stainless steel and comparatively high thermal diffusivity and conductivity (Ref 2, 3). This at present reduces cost-effectiveness due to inevitable boil-off and disregards some other requirements such as mechanical and cyclic strength and high corrosion resistance (Ref 1, 4). Therefore, thermal barrier coatings (TBC) applied by thermal spraying are becoming the focus of attention due to their high insulating effect and wear resistance at higher temperatures. As a working hypothesis, it is assumed that TBC can counteract boil-off and additionally protect the inner tank shell from dynamic sloshing forces in cryogenic environments too.
Arc spraying is a simple and cost-effective thermal spray technology, which is determined by a few key factors (Ref 5, 6). Arc voltage, current and gas flow (e.g., free, forced etc.), pressure, and type mainly affect coating quality (Ref 5, 7). Besides, residual stresses in the coatings are strongly influenced by these parameters and the spray kinematics like the stand-off distance, spray pattern, robot speed, etc. (Ref 7, 8). In general, residual stresses in thermal spray coatings originate from the superposition of quenching, cooling, and other stresses, generated during spraying and afterward (Ref 9,10,11). Quenching stresses mostly occur in high thermal energy technologies, which is the case for arc spraying. Initiated by particle impingement during the coating, this stress component usually appears to be tensile. The particles cannot contract freely in the very short cool down phase from the melting temperature due to the substrate surface (Ref 9, 10, 12). On the other hand, cooling stresses emerge from the thermal mismatch between coating and substrate after spraying, which is characterized by the usually different coefficients of thermal expansion (Ref 9, 11, 12). Hence, the cooling stresses can only roughly be estimated in terms of their tensile or compressive nature mostly (Ref 13).
Since arc spraying is already established, e.g., for corrosion protection of large structures, the technology is also considered for preparing metallic TBC on large inner tank hulls. For this purpose, conventional Ni-based (Ref 13, 14) and novel amorphous Fe-based (Ref 15,16,17) alloys seem to be promising candidates due to potentially suitable thermal and mechanical properties, i.e., low thermal conductivity and fracture toughness. However, the influence of cryogenic conditions on the durability of the coatings due to the residual stresses, cracks, and pores is possibly challenging due to inevitable sloshing mechanisms and dynamic forces (Ref 1, 4) and not yet fully known. In detail, residual stresses in coatings under such loads and with correspondingly adjusted porosities could lead to premature coating delamination. Therefore, the consequences of these special circumstances remain to be investigated in detail and shall be one focal point of the study, while the other main aspect will be the thermal insulation capability. A key factor influencing the thermal diffusivity and conductivity is the porosity of the coatings. In the present work, a typical NiCrAlY alloy and, moreover, amorphous Fe-based alloys are arc-sprayed due to the combination of high cost efficiency, easy process handling, and customizable coating properties. In particular, the NiCrAlY alloy was selected because it is state of the art as a bond coat in the field of TBC and at the same time has promising potential properties. In contrast, the selected amorphous alloy with high hard material content and the partly amorphous pseudo-alloy represent a new material approach, which are characterized by potentially good insulation properties and economic advantages such as high deposition rates.
Previous own works for other applications and further literature revealed that coatings sprayed with nitrogen or a mixture of nitrogen and hydrogen show superior coating qualities with respect to oxidation and electrical conductivity (Ref 5, 18)Thus, nitrogen is used as process gas, while the stand-off distance and the number of passes were varied to modify the porosity in a targeted manner. Further properties investigated besides the main focal points regard hardness, tensile adhesive stresses, and electrical conductivity. The latter could provide a nondestructive testing method, since a causal relationship to the coating defects is known.
Experimental Methods
Spray Process, Materials, and Deposition Efficiency
Arc spraying experiments were performed by using a DURUM Durspray 450 (DURUM Verschleißschutz GmbH, Willich, Germany) equipped with an ASJO2nd torch (T-Spray GmbH, Lenningen, Germany). Three different materials were arc-sprayed. The first wires used were of type DURMAT® AS-777, which corresponds to a composition of NiCr22Al10Y1. The next material was DURMAT® AS-812, which represents amorphous alloy of composition FeCr30B3.9Si1.0Mn0.7. Finally, wires of type DURMAT® AS-SNA-1090 with a composition of FeCr28Ni7B3.5Mo1.5MnSi were utilized realizing a combination of austenitic phases corresponding to 316L and amorphous phases (all wires supplied by DURUM Verschleißschutz GmbH, Willich, Germany; diameters of 1.6 mm). The substrates were of type 1.4301 hot-rolled steel with dimensions of approx. 58 × 50 × 8 mm3. Prior to spraying, the substrates were grit-blasted using corundum and finally cleaned by using isopropyl alcohol.
The constant parameters including the kinematics of the experiments can be found in Table 1. Moreover, the varying parameters and desired thicknesses can be found in Table 2 along with the resulting measured coating thickness and the deposition efficiency (DE). While the DE was specified following ISO 17836, the coating thickness was determined in cross sections following the procedure described hereafter in the section “Microstructure.” The spray pattern used corresponded to a meander-shaped type carried out by a robot, while the specimens were fixed. From here on, the coating materials shall be referenced using the abbreviations given in Table 1. If other materials are intended, e.g., bulk materials, this will be made explicitly clear.
Tensile Adhesive Strength
Coating adhesion was investigated by tensile adhesive strength tests according to ISO 14916 by using a static uniaxial testing machine (Zwick Roell Z50, Ulm, Germany) with three specimens each (Ø 25 × 25 mm). The specimens and counter bodies were glued by using HTK Ultrabond 100 adhesive (HTK Hamburg GmbH, Hamburg, Germany) and subsequently cured at 180 °C for at least 50 min. The material used was also 1.4301. Mean and standard deviation were then formed only from specimens which failed in the coating, as required by the standard.
Hardness
Hardness was measured on ground surfaces as HV 10 by using a Wolpert 432 (Wilson Wolpert Instruments, Aachen, Germany; applied load 98 N). Fifteen measurements were taken for each specimen in accordance to ISO 6507-1. Subsequently, the ratio of diagonals of the imprints was checked to achieve proper imprints. Thus, only the seven imprints having the diagonals with the least deviation from each other were chosen. Finally, the minimum and maximum values of these seven measurements were deleted and not included in the further calculation—resulting in a total of five sufficient measurements. The described procedure is in accordance with (Ref 14).
Microstructure
For microstructural analyses, the specimens were first cold-mounted (two-phase system: liquid hardener and powder resin) and gradually ground and polished (6 µm, and 3 µm suspensions, finally oxide polish). Coating thickness was determined by using an optical microscope (OM) Leica DM6000M (Leica Microsystems GmbH, Wetzlar, Germany) and the software tool ImageAccess (Imagic Bildverarbeitung AG, Glattbrugg, Switzerland) recording 3 times 7 measured values, while eliminating maxima and minima values. Further, a scanning electron microscope (SEM) JEOL JSM-IT100 (JEOL Germany GmbH, Freising, Germany; magnification ×1000, acceleration voltage 10 kV, backscatter detector, low vacuum mode) and the software ImageJ (National Institutes of Health, USA; in region of interest using Despeckle filter, normalization and finally Trainable Weka Segmentation tool) were used for examining the content of porosity/oxidation/cracks inside the coatings at 3 areas inside each specimen. Moreover, representative analyses regarding the morphology were carried out by using the same SEM at different magnifications (×500 and ×2000). Furthermore, energy-dispersive x-ray spectrometry (EDS) was executed within the cross sections in the same places by using a JEOL Dry SD25 detector (JEOL Germany GmbH, Freising, Germany; acceleration voltage 15 kV, high vacuum mode, magnification 2000x). By this, the local chemical composition of the coatings was identified in the same places as the representative analyses regarding the morphology. From the interaction between contrast by SEM, in particular possible dendritic formation, as well as chemical composition from EDS, the amorphous and crystalline regions should be well distinguishable well from each other.
Electrical Conductivity
The specific electrical conductivity was determined using the four-terminal measuring method (Loresta GX MCP-T700, Mitsubishi Chemical Analytech Co. LTD, Kanagawa, Japan) by investigating 2 distinct areas with 7 values each for a coated substrate of the corresponding parameter (2 coated specimens per parameter of Table 2) and for the bulk substrate material (also 2 specimens, but not coated). In this method, the determined ohm resistivity is converted into the specific resistivity by multiplication with a correction factor RCF (depending on geometry and measuring position) and the corresponding coating thickness or, as an inverse value, into the electrical conductivity. The corresponding RCF was determined individually for each sample. Before measuring in the procedure described above, optimum measurement range and current were determined by an own developed computer routine via scanning different parameters. This enables more accurate measurements. Furthermore, the insulating base Resi Table MCP-ST03 (Mitsubishi Chemical Analytech Co. LTD, Kanagawa, Japan) is used as ground to reduce impact of leakage currents and external influences on the results. The test method allows for measuring the electrical properties in as-sprayed and as-polished states.
Cryogenic Experiments
A cold gas system Isotherm TG-L63/100 (KGW-Isotherm Karlsruher Glastechnisches Werk–Schieder GmbH, Karlsruhe, Germany) with a vacuum-insulated stainless steel cryostat was used for the cryogenic experiments. An evaporator and counter heater with thermocouples allowed for exact temperature control. Liquid nitrogen was used for cooling, which was carried out in a first step at − 100 °C for 2 h and later at − 150 °C for 2 h. Afterward, the specimens were allowed to warm up to room temperature while condensing moisture was removed with compressed air. Subsequently, examination via SEM (same device and parameters as in section “Microstructure”) occurred. After both cooling sessions, the same areas of the samples were investigated as before cooling to identify possible effects on the coatings. The coatings investigated were of target thickness 500 µm and SOD of 75 mm (see Table 2) and consisted of as-sprayed specimens as well as microsections (preparation according to section “Microstructure”).
Residual Stress Analyses
Residual stress analyses of the coatings were carried out by using a Stresstech PRISM system utilizing electronic speckle pattern interferometry (ESPI) and the PrismS software (both Stresstech GmbH, Rennerod, Germany) with one representative measurement for every material as well as an SOD of 75 mm and a thickness of 500 µm each. The coatings were tested in the as-sprayed state after the application of a developer spray (typically used in the non-destructive penetration test) to reduce surface reflections and achieve good lighting. The holes were processed in varying incremental steps using coated high-speed drill flute mills (Gühring SL WN K VHM Ø 0.8 mm FIRE, Albstadt, Germany) up to a final profile depth of 350 µm. The inner integration radius was 3 times the hole diameter, while the outer integration radius corresponded to 5 times the hole diameter. The elastic parameters for stress calculation corresponded to a Poisson ratio of 0.325 for all of the coatings, while Young’s moduli were set to 85 GPa for Ni-base (average value from (Ref 19)) and 181 GPa for the amorphous/partly amorphous coatings, respectively (average value from (Ref 16)). A Tikhonov regularization factor of 0.01 was applied in stress calculation. In addition, the integral below the stress curves (as sum of tensile and compressive amounts) was calculated numerically by trapezoidal integration using an in-house MATLAB routine. Due to its nature, this integral shall further on be referred to as the cumulative stress.
Thermal Diffusivity
Thermal diffusivity was measured using LFA 467 HT HyperFlash (Erich NETZSCH GmbH & Co. Holding KG, Selb, Germany) on specimens of a size of 10 × 10 mm2 in a temperature regime from 25 to 300 °C. Due to the missing opportunity of measuring in cryogenic environment, this regime is as close as possible to low temperatures and delivers a good impression of the insulating behavior. The gas used was argon and the properties assumed, especially density, were of type stainless steel as was the substrate. The specimens were matted by using graphite spray.
Originally, it was planned to separate the coatings from the substrate at the interface by means of an impact test (INSTRON–CEAST 9340, Pianezza, Italy) to be able to investigate the pure coating properties. However, since this was not possible even at 20 J (see Fig. 1), the substrate was subsequently ground down as far as possible. The total thicknesses of the specimens for measuring were 1.170, 1.182, and 1.162 mm for the investigated samples of target thickness 500 µm and SOD of 75 mm (see Table 2). This low thickness in comparison with the original substrate height of 8 mm is the minimum, which can be handled by grinding manually. This ultimately means that the ratio of remaining substrate to coating is still roughly about 2.6-2.8 to 1. The ratio must be considered in the evaluation of the samples. In addition, the thermal diffusivity was also determined for the bulk substrate material.
Results
Direct Coating Characterization
The typical appearance of the as-sprayed specimens can be found in Fig. 2. At first glance, all surfaces look very similar metallic bright. However, the Ni-base samples partially showed staining, which indicates higher heated areas. Moreover, with increasing coating thickness a tendency toward growing occurrence of coating protrusions/splatters became apparent. The partly amorphous Fe coatings revealed the most regular surfaces overall.
When comparing the target values of coating thickness to the actual measured values (see Table 2), it can be observed that in particular 100 µm and 300 µm were achieved relatively well. In contrast, the target thickness of 500 µm was achieved only for Ni-based materials, while the same number of passes for the Fe-based materials resulted in measurements of rather 400 µm. Anyway, a more precise adjustment in the arc spraying process, e.g., by changing the number of passes or wire feed rate, was hardly possible due to the nonlinear process and coating characteristics. This had a particularly strong effect with higher coating thicknesses.
Regarding the DE, it is evident that a higher spray distance leads to lower values, as expected (see Table 2). Likewise, a higher coating thickness, except for the partially amorphous Fe coatings for an SOD of 125 mm, leads to an increase of DE. Furthermore, the values are all below the manufacturer’s specifications according to the data sheet. However, this is not very surprising since, firstly, nitrogen was used exclusively as the process gas and, secondly, the DE was calculated based on the normal coated flat substrates and not on specially provided test specimens.
Adhesive and Mechanical Properties
Figure 3 shows the values for the adhesive tensile strength. A decrease can be observed for each material with increasing coating thickness for both SOD. In percentage terms, however, these are somewhat lower for higher SOD. In a combined comparison of SOD and coating thickness, Ni-base almost always shows the highest values, while the lowest adhesive tensile strengths can be observed for Fe partly amorphous. Nevertheless, a sufficiently high adhesion is given for all materials, although there is some scattering in parts. A certain scatter is also observable for the uncoated substrate, while the numbers of the coatings are lower predominantly for Fe partly amorphous.
The hardness of the coatings can be found in Fig. 4. For the thinnest coatings, significantly lower values can initially be found compared to the coatings with higher thicknesses, especially for the amorphous and partly amorphous alloys. Furthermore, for the thicker coatings, it can be observed that a decrease tends to occur with an increase in SOD for Ni-base and Fe-amorphous. The highest values, but also scatter, are found for Fe-amorphous, while the lowest hardness is observed for Ni-base. The values for Fe-partly amorphous are above Ni-base, but significantly below Fe-amorphous. However, on the other hand the most constant hardness is to be seen for Fe-partly amorphous, i.e., the numbers for the thinnest coatings are roughly the same which also accounts for the values of thicknesses 300 and 500 µm (all around 300 HV10).
Representative Investigation of Coating Microstructures
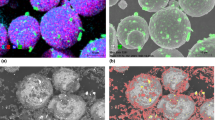
Figure 5 shows the typical microstructures of the coatings. Basically, all coatings show a lamellar structure typical for arc spraying, although the lamellae sizes vary considerably in some cases. Similarly, all coatings are well bonded to the substrate. For Ni-base, some porosity and partial non-uniform oxidation can be seen (see SEM detail image in Fig. 5(a)). In addition, there are some very bright areas in the OM image. For Fe-amorphous only little oxidation is visible, rather hard phases are to be assumed by comparison of SEM and OM images and especially SEM detail image in Fig. 5(b). On the other hand, the formation of cracks in the coatings is clearly visible for this coating type. Furthermore, as desired, practically no crystallization is visible for Fe-amorphous, in contrast to the other sprayed materials. On the other hand, differently contrasted amorphous areas can be observed, especially in the SEM image. For Fe-partly amorphous in Fig. 5(c), it can be seen well that both amorphous and classical crystalline regions occur about half each. In addition, irregularly shaped oxidation and clustered porosity is evident. Cracking, however, does not occur for Fe-partly amorphous, while some hard phases can be identified in the material (see SEM detail image).
Representative morphology of the coatings for all three materials examined by OM (top) and SEM (middle) as an overview and by SEM in detail (bottom) for materials (a) Ni-base, (b) Fe-amorphous and (c) Fe-partly amorphous. The yellow dots in the SEM detail images show the locations where EDS was performed as a spectrum to detect local differences in composition. (d) Chemical composition of the areas by EDS spectra corresponding to the yellow dots. The abbreviation “n.d.” stands for not detectable
The described characteristics are confirmed by the EDS spectra, the results of which are summarized in Fig. 5(d). The corresponding measuring points are shown in the SEM detail images in Fig. 5(a), (b), and (c) for all sprayed materials. Thus, the clustered dark areas for Ni-base in Fig. 5(a) are more likely to be complex compounds or oxides, mainly of Al, O, and Y and C (cf. P3). In contrast, P1 represents the usual composition of the Ni-base alloy, although some burn-off of Al and Y is evident compared to the wire composition. Point P2 is predominantly characterized by Ni, while other similarly bright areas from the OM images turned out to be hard phases (not shown due to partly redundant information other than the hard phases). With respect to Fe-amorphous, it should be noted that the superposition of the light element B peak with C complicates the evaluation. However, together with other analyses, P4 was found to correspond to a hard phase with B, C, Si, and Cr (carbides, carborides) superimposed by cracks. In contrast, P5 corresponds to the composition of Fe-amorphous with few burn-off losses in terms of Cr and Si. For P6, no excessive excess of O can be observed, the composition essentially corresponding to P5, although some influence of the sample drift cannot be excluded here.
Significant differences are observed for Fe-partly amorphous. Thus, P7 is in the crystalline range of the material, which is also reflected in the composition, which essentially corresponds to a high-alloy stainless steel. For P8, on the other hand, a more amorphous composition is recognizable. Furthermore, P9 corresponds to a hard phase with high contents of B, C, and Cr (carborides). The clustered porosity of P10 contains oxygen-affine elements such as Al together with O itself.
Quantitative Coating Analyses and Electrical Properties
In Fig. 6, the sum of defects (porosity, oxides, and cracks, cf. section “Microstructure”) is displayed. For Ni-base, a significant defect content is evident, which is diminished by increasing coating thickness. Furthermore, a higher defect level can also be observed here at higher SOD. In contrast, the amorphous materials all exhibit a similarly constant, significantly lower defect content, which is only higher in two out of three cases for a higher SOD. It should also be noted that the observed cracks of Fe-amorphous are of little significance and, in general, the scatter is slightly higher for thicker coatings of these materials. A clear dependence on growing coating thickness on the other hand is only visible for Fe-partly amorphous and an SOD of 75 mm showing a rise in defects.
The electrical conductivity can be found in Fig. 7. It is generally distributed in a narrow regime for all samples and differs significantly from the measurements on the uncoated substrate (1.58 ± 0.07 106 S/m). For Ni-base, a significant decrease with increasing coating thickness is observed for both SOD. Furthermore, the conductivity is always higher for this coating material for higher SOD. For Fe-amorphous, on the other hand, no dependence on the parameters or thicknesses is observed—considering the standard deviations, all values are in the same narrow range. For Fe-partly amorphous, a decrease in conductivity with thickness is observed for the lower SOD, but not for the higher SOD. For the latter, the numbers are like those for Fe-amorphous.
Cryogenic Durability
Figure 8 shows representative for all coatings the variant of Fe-partly amorphous with an SOD of 75 mm and a target thickness of 500 µm under the influence of the different temperature regimes. This variant was selected because it contains both amorphous and crystalline phases and thus also represents the other materials well. In addition, the observations described below apply to all coatings.
First, it should be noted that neither exposure cycle resulted in any changes on the microstructural level of the specimens. In Fig. 8(a), isolated artifacts due to reheating and condensation of moisture can be recognized. Furthermore, ice crystals were detected on some microsections despite the rapid cleaning with compressed air, which, however, only adhered to the surface. Figure 8(b) illustrates that hardly any changes were noticed for the surfaces of the coatings either. Occasionally, only very fine, loosely bound particles were detached from the surface, but this did not affect the function of the coatings.
Residual Stress States
Figure 9 illustrates the representative residual stress trends over the depth profile. It is obvious that the three materials show completely different trends. For Ni-base, slight, almost constant tensile stresses are present throughout the coating. For Fe-amorphous, on the other hand, the stress levels oscillate around the zero value with quite large fluctuations. A clear statement as to whether tensile or compressive stresses dominate is therefore not possible. For Fe-partly amorphous, the highest stress values can be detected, which are particularly noticeable at greater depths. This is followed by a change of sign to compressive stresses, with high fluctuations from tensile to compressive being observed for this material.
The observations on the curves are supported by the cumulative stresses in Fig. 10, in which it is evident that moderate tensile stresses are present for Ni-base. In contrast, for Fe-amorphous, an almost stress-free state with a cumulative stress close to zero is apparent. The highest value can be identified for Fe-partly amorphous, which is almost twice as high overall as for Fe-amorphous.
Insulating Behavior
Figure 11 shows the thermal diffusivities of the coatings compared to the uncoated substrate. Ni-base shows the highest values of the three coating materials, which grow with increasing temperature. The diffusivity of the substrate, however, is clearly above the coatings with an average factor of 1.6. Similarly, a slight bend in the curve of the lower temperatures is seen for both the substrate and Ni-base. The same observations hold true in principle for Fe-amorphous, although the values are still a little lower than for Ni-base and the curve appears somewhat more linear.
For Fe-partly amorphous, on the other hand, a clear difference in the level of the values can be seen. The material exhibits the lowest diffusivities, which are lower by a factor of 2.3 on average compared to the uncoated substrate. Also, the overall slope of the curve seems to be lower than for the other materials.
Discussion
Findings on Process, Adhesion, and Mechanical Properties
The coatings investigated in this work were produced regarding certain target properties, in particular stability under cryogenic conditions and thermal insulation capability. At the same time, however, the application of the coatings is linked to other properties, some of which have not yet been considered at all or only to a limited extent. Furthermore, economic efficiency also plays a role, of course.
Therefore, process characteristics such as the deposition efficiency, the adjustable coating thicknesses, and the surfaces (i.e., potential post processing) were considered first. The surfaces showed a regular, overall well machinable, surface with some staining indicating in part strong heat input for Ni-base. The Fe partly amorphous coatings, on the other hand, showed the most uniform surface even at higher thicknesses, which can probably be attributed in part to the different phase formation. This probably allows for different particle sizes in coating build-up and tends to counteract the typical roughening with increasing thickness. Despite the use of nitrogen and the suppression of excessive oxidation, the deposition efficiency was lower than assumed according to the manufacturer's specifications. This is, however, still acceptable, especially considering the determination on the standard flat specimens. Furthermore, the exact adjustment of the thickness was slightly more difficult with amorphous materials than with conventional materials, as can be seen in the comparison with Ni-base. This, too, can certainly be attributed to the changed coating formation and residual scattering of the used power source. Overall, however, the Ni-base and amorphous coatings can be applied economically.
Another important property is the adhesion of the coatings, especially considering the challenging conditions for the planned application. Here, the coatings showed high values, ranging from 30 to approx. 70 MPa. While the slightly lower values for higher SOD are plausible due to the changed impact conditions, it is not entirely clear where the drop with increasing coating thickness originates from, especially in the case of the amorphous materials. It is conceivable that the interparticle bonding due to the increasing inhomogeneity of the coatings (porosity/cracks/oxidation, but also hard phases) plays a role in this. Another fact that speaks for the generally good bonding is further visible in the fact that a detachment at the interface by means of an impact test was not possible. However, the scatter for the uncoated substrate reveals that the adhesion process needs to be adjusted to compensate for the changed viscosity for future investigations. Although the hardness is only suitable to a limited extent for depicting the expected load regime, it can serve as a first orientation about the strength of the coatings. It is evident that all the coatings investigated so far show sufficient values, especially the thicker ones. As expected, the Fe-amorphous coatings show the highest hardness due to the increased content of hard phases in the microstructure. The partly high scatter can also be attributed to this. However, for thinner coatings, the hardness test on the sample surface seems to be increasingly subject to the influence of the substrate. The lower levels compared with the manufacturer's specifications can be attributed in part to the limitations on the part of the hardness tester (no differentiation between matrix and hard phases possible). On the other hand, previous own investigations showed hardness losses when using nitrogen as process gas for materials with oxygen affinity elements (Ref 18).
Insights into Microstructure and Functional Properties
The microstructure was presented by correlative microscopy. The approach used showed differences in the imaging capability and in the interpretation of chemical compounds. For example, some black areas which could be interpreted as porosity by OM are more likely to be identified as complex compounds in SEM/EDS. Certain carbides, on the other hand, can be detected more quickly with OM than with SEM. For that reason, SEM images were evaluated for quantitative coating analyses, while a good first impression in terms of quality was given by comparing SEM and OM. A very regular, mostly lamellar morphology with a very low porosity and oxidation content was achieved, which could be manipulated by the process parameters. The use of nitrogen and the shroud effect (Ref 5) thus provide a high coating quality by effectively shielding the particles from the atmosphere. The phase composition achieved corresponded as planned to a combination of amorphous, crystalline, and hard phases in case of the (partly) amorphous alloys and moreover a classic alloy in terms of Ni-base. The amorphous coatings showed lower percentages of defects than Ni-base, while cracking was especially visible for Fe-amorphous. This serves to relieve residual stresses and is intended in this amorphous material used. In this way, thick, stress-free coatings can be applied, which should have a positive impact on long-term durability under cryogenic conditions. The cracking was therefore expected.
The tensile stresses for Ni-base and even higher values for Fe partly amorphous are plausible in principle. With respect to the latter, the internal material transition between amorphous and crystalline splats plays a major role, since the material models used for the stress calculation are always based on a single material only. However, while Ni-base showed good measurability, this only holds true to a certain degree for the amorphous and partly amorphous alloys. This, in part, can be attributed to the drill flute mills used, which are only to a limited extent suitable for coatings with a high hard phase content. It should also be noted that the characteristic elastic parameters were taken from the literature and scale the results accordingly. Although the absolute values of the stresses can thus not be assumed to be one hundred percent correct, the overall trends are generally reasonable. For example, the pure amorphous material was found to be almost stress-free in average, which fits well with both the assumptions made in advance and the results of the microstructural investigations. In particular, this refers to stress relief due to crack formation. In short, the stress state does not seem to have a major influence on the adhesion of the coatings, even under cryogenic conditions.
If the values in Fig. 6 and 7, i.e., defect content and electrical conductivity, are correlated, different trends become apparent. For Ni-base, a decreasing content of pores and oxides obviously correlates with a lower conductivity (i.e., higher resistivity as reciprocal), which in some cases also drops significantly below the value of the uncoated substrate. For the amorphous materials, on the other hand, a minor dependence can only be observed for Fe-partly amorphous at an SOD of 75 mm. Here, however, a lower conductivity correlates with an increasing defect content. Even if this seems contradictory at first, own investigations in the past showed that NiCr-based materials are associated with oxidation (Ref 20). The oxidation, in this case also of Al and Y, might result in various compounds changing the conductivity of the overall coating. On the one hand, it must be considered that it is difficult to distinguish between oxidation and porosity when determining the defects automatically. On the other hand, the defects for Ni-base are oxidation-dominated still, as was evident from the microstructures, so that the influence of any chemical compounds on the conductivity is higher than for the other materials. In contrast, for materials with good conductivity, such as pure Cu, a higher conductivity is observed with decreasing defects (Ref 21). The results are thus plausible. At the same time, however, they also show that a higher proportion of crystalline phase could be present for this special variant of Fe-partly amorphous. This is particularly evident in direct comparison with the values of Fe-amorphous, which are constant regardless of the parameters used and the coating thickness. It can thus be indirectly concluded that, on the one hand, a higher proportion of amorphous phase is present at an SOD of 125 mm and, on the other hand, that the measurement methodology is suitable for nondestructive testing of these materials. It must be noted, however, that the conductivity for Fe-partly amorphous and a SOD of 75 mm scatter considerably in some cases and the statement must therefore be regarded as preliminary. These observations demonstrate that the structure of the coatings with the contradictory mechanisms of nucleation and amorphous formation, in addition to the composition of the alloys, is very strongly dependent on the parameters used. The differences in enthalpy, particle characteristics, and cooling behavior are in part so small that a slight change in kinematics leads to different coating behavior, cf. Table 1.
Another result of this work is that it could be shown that cryogenic storage has no visible effect on the microstructure of thermally sprayed coatings, even though substrate and coatings have different coefficients of thermal expansion. Although some loosely bound particles detached after this procedure, this is not important for the application, since the coatings will probably be ground or grit-blasted. Finally, the investigations of the thermal diffusivity showed very promising results. The measured values were significantly lower than for the pure substrate despite the low coating thickness and a ratio of 2.6-2.8 to 1 from substrate to coating thickness. The influence of the substrate can thus be described as substantial and needs to be reduced. In particular, very low values were found for Fe-partly amorphous and the SOD of 75 mm, which is probably due to the combination of amorphous and crystalline phases. These additional transitions in the material probably increase the inertia of the material with respect to temperature and fit well with the observations made with respect to porosity and electrical conductivity.
Summary and Outlook
In this work, a NiCrAlY alloy and amorphous Fe-based alloys were arc-sprayed for applications in cryogenic environments, especially for structures with high desired insulation capability such as LH2 tanks. Nitrogen was used as process gas, while the stand-off distance and number of passes were varied. Promising coating properties were achieved, especially for Fe-partly amorphous which combined the best of the two material approaches, proven by electrical conductivity and porosity measurements as well as thermal diffusivity. In addition, it was shown that the cryogenic stress did not harm the coatings and that even the partly high tensile residual stresses had no effect on the adhesion of the coatings.
The further focus will be to be able to determine the direct coating properties such as the thermal diffusivity through targeted delamination by means of surface pretreatments of the substrates. If the promising results in this respect are confirmed over a longer period and under load, the use of such coatings is conceivable not only in LH2 tanks but also in numerous other cryogenic applications.
References
Frost and Sullivan, “Disruptive Innovations in Production, Storage, and Transportation of Hydrogen, Market Report”, Santa Clara (US-CA), 2020
M. Klell, H. Eichlseder, and A. Trattner, Hydrogen in Automotive Engineering: Production, Storage, Application, Springer, 2023.
M. Hirscher Ed., Handbook of Hydrogen Storage: New materials for future energy storage, Wiley, 2010
H. Pinto, A. Pyzalla, R. Büscher, A. Fischer, K. Aßmus, and W. Hübner, The Effect of Hydrogen on the Deterioration of Austenitic Steels During Wear at Cryogenic Temperature, Wear, 2005, 259(1-6), p 424-431.
F. Lang and W. Krömmer, Economic and Ecological Benefits of Using Gas Mixtures for Arc Spraying, Tagungsunterlagen 10 Kolloquium Hochgeschwindigkeits-Flammspritzen/10Th Colloquium Hvof Spraying: 29. Und 30. Oktober 2015, Erding, Conference Proceedings, C. Penszior, Ed., Gemeinschaft Thermisches Spritzen e.V, 2015, p 79-88
J.-H. Kim and M.-H. Lee, A Study on Cavitation Erosion and Corrosion Behavior of Al- Zn-, Cu-, and Fe-Based Coatings Prepared By Arc Spraying, J. Therm. Spray Technol., 2010, 19(6), p 1224-1230.
J. Chi, A. Zhang, S. Xie, and C. Jin, Process Optimization and Residual Stress Measurement For Arc Spraying Rapid Tooling, J. Xi’an Jiaotong Univ., 2014, 48(12), p 126-130.
M. Hauer, K.M. Henkel, S. Krebs, and W. Kroemmer, Study of Traverse Speed Effects on Residual Stress State and Cavitation Erosion Behavior of Arc-Sprayed Aluminum Bronze Coatings, J. Therm. Spray Technol., 2017, 26(1-2), p 217-228.
S. Sampath, X. Jiang, J. Matejicek, L. Prchlik, A. Kulkarni, and A. Vaidya, Role of Thermal Spray Processing Method on the Microstructure, Residual Stress and Properties of Coatings: an Integrated Study For Ni-5 Wt.%Al Bond Coats, Mater. Sci. Eng. A, 2004, 364(1-2), p 216-231.
S. Kuroda, T. Fukushima, and S. Kitahara, Significance of Quenching Stress in the Cohesion and Adhesion of Thermally Sprayed Coatings, JTST, 1992, 1(4), p 325-332.
J. Pina, A. Dias, and J. Lebrun, Study by X-Ray Diffraction and Mechanical Analysis of the Residual Stress Generation during Thermal Spraying, Mater. Sci. Eng., A, 2003, 347(1-2), p 21-31.
T.W. Clyne and S.C. Gill, Residual Stresses in Thermal Spray Coatings and Their Effect on Interfacial Adhesion: A Review of Recent Work, J. Therm. Spray Technol., 1996, 5(4), p 401-408.
L. Pawłowski, The Science and Engineering of Thermal Spray Coatings, 2nd ed. Wiley, 2008.
H.-A. Mathesius and W. Krömmer, Praxis Des Thermischen Spritzens: Anleitung für das Fachpersonal. In German, 2nd ed., DVS Media, 2014
W. Guo, Y. Wu, J. Zhang, S. Hong, G. Li, G. Ying, J. Guo, and Y. Qin, Fabrication and Characterization of Thermal-Sprayed Fe-Based Amorphous/Nanocrystalline Composite Coatings: an Overview, J Therm Spray Tech, 2014, 23(7), p 1157-1180.
K. Bobzin, M. Öte, and T. Königstein, Manufacture of Iron-Based, Amorphous Coatings With High Fracture Toughness, IOP Conf. Ser. Mater. Sci. Eng., 2017, 181, p 12012.
K. Bobzin, M. Öte, and T. Königstein, Investigation of Amorphous/Nanocrystalline Iron-Based Thermal Barrier Coatings, J. Therm. Spray. Technol., 2017, 26(3), p 388-397.
M. Hauer, K.M. Henkel, S. Krebs, and W. Kroemmer, Alternative Gas Mixtures in Arc Spraying: a Chance to Improve Coating Properties and Residual Stress States, J. Therm. Spray Technol., 2018, 27(1-2), p 106-118.
T. Keller, “Pore morphology in thermally sprayed nickel-based deposits and the influence on deposit properties”, ETH Zurich, 2003
M. Hauer, B. Ripsch, W. Krömmer, and K.-M. Henkel, Use of Alternative Process Gases and Modifications For Manufacturing Heating Elements By Arc Spraying, New Waves of Thermal Spray Technology For Sustainable Growth: International Thermal Spray Conference and Exposition (Itsc 2019) Yokohama, Japan, 26-29 May 2019, F. Azarmi, Ed., Curran Associates Inc, 2019, p 504-511
M. Hauer, R. Banaschik, and H. Knuth-Michael, Innovative Quality Assurance of Laser Welded Joints on Arc-Sprayed Coatings Using Alternative Gas Mixtures, Tagungsunterlagen 11 Kolloquium Hochgeschwindigkeits-Flammspritzen/11Th Colloquium Hvof Spraying: 25. Und 26. Oktober 2018, Erding, Conference Proceedings, C. Penszior, Ed., Gemeinschaft Thermisches Spritzen e.V, 2018, p 111-122
Acknowledgments
The authors would like to thank all co-workers involved in the study, namely listed in alphabetical order: P. Andreazza, D. Kelm, F. Knöchelmann, D. Krüger, V. Nikolova, N. Peters, S. Schneider, and P. Wiechmann. The IGF project 39 LBR/1 of the research community center “Forschungsvereinigung Stahlanwendung e.V.—FOSTA” (Sohnstraße 65, 40237 Düsseldorf, Germany) is funded by the German Federal Ministry of Economic Affairs and Climate Action through the AiF within the framework for promotion of joint industrial research (IGF) in the overall project entitled “Offshore Wind Energy Systems for Hydrogen Supply” based on a resolution of the German Bundestag. The project is carried out at Fraunhofer IGP. We would like to thank the sponsors, cooperating research institutes and the participating companies.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is an invited paper selected from presentations at the 2022 International Thermal Spray Conference, held May 4–6, 2022 in Vienna, Austria, and has been expanded from the original presentation. The issue was organized by André McDonald, University of Alberta (Lead Editor); Yuk-Chiu Lau, General Electric Power; Fardad Azarmi, North Dakota State University; Filofteia-Laura Toma, Fraunhofer Institute for Material and Beam Technology; Heli Koivuluoto, Tampere University; Jan Cizek, Institute of Plasma Physics, Czech Academy of Sciences; Emine Bakan, Forschungszentrum Jülich GmbH; Šárka Houdková, University of West Bohemia; and Hua Li, Ningbo Institute of Materials Technology and Engineering, CAS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hauer, M., Gericke, A., Möhrke, L. et al. Highly Efficient Thermal Barrier Coatings Based on Arc Spraying of Amorphous Fe-based Alloys and NiCrAlY for Use in LH2 Tanks and Other Cryogenic Environments. J Therm Spray Tech 32, 327–338 (2023). https://doi.org/10.1007/s11666-023-01548-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-023-01548-8