Abstract
The present study deals with the development and characterization of Mo-35Ti-10Si and Mo-35Ti-10Si-2B (wt.%) alloy for ultra-high temperature applications beyond the temperature limit of existing super alloys. The microstructural characterization using scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), electron back scattered diffraction (EBSD), x-ray diffraction (XRD) revealed that the Mo-35Ti-10Si-2B alloy was consisted of three phases, namely, (Mo, Ti)ss, (Mo, Ti)5SiB2 and (Ti, Mo)5Si3; whereas, Mo-35Ti-10Si alloy was found to be consisting of (Mo, Ti)ss, and (Mo,Ti)3Si phases. Since quantification of boron is difficult by EDS, Particle Induced Gamma-ray Emission (PIGE), a nuclear reaction analysis technique was used for chemical composition analysis of boron. The oxidation behavior of the Mo-35Ti-10Si-2B alloy in the temperature regime of 825-1250 °C was studied in detail and compared with boron-free Mo-35Ti-10Si alloy. Mo-35Ti-10Si-2B alloy exhibited superior oxidation behavior at intermediate temperatures of 825 °C, and excellent oxidation resistance at higher temperatures between 1000 and 1250 °C due to the formation of the protective borosilica and double oxide layers (TiO2 and duplex borosilica-TiO2), respectively. High-temperature oxidation mechanisms were discussed using detailed microstructural cross section analysis of the oxidized alloy samples. The micro-mechanical behavior of constitutive phases of the Mo-35Ti-10Si-2B alloy were studied by microhardness, nano-indentation and micropillar compression testing. The micropillar compression of (Mo, Ti)ss phase showed fairly ductile behavior with the evidence of activation of dislocation in the form of slip lines revealed through the post-deformation fractography. Deformation studies of (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phases were also carried out which showed large strain bursts indicating possibility of activation of dislocation activities even at room temperatures imparting low level of ductility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Substantial progress has been made in the development of multi-phase molybdenum-based alloys such as Mo-Si-B, Mo-Ti-Si which has shown progressive improvements with respect to high temperature properties and recommended to be among the potential candidates for ultra-high temperature applications (Ref 1,2,3,4,5,6,7,8). Typically, Mo-Si-B-based alloys comprise of Mo solid solution (Moss) as the phase responsible of imparting room temperature toughness and Mo5SiB2 (T2) as the oxidation resistant phase (Ref 1, 2, 5). This has been shown and well documented that presence of boron in the T2 phase helps in the formation borosilicate passivating layer by decreasing its viscosity (Ref 2). Apart from Moss and Mo5SiB2 (T2) phases, either Mo5Si3 or Mo3Si phases are also present in the Mo-Si-B-based alloys depending on the Si concentration in the alloy. Mo5Si3 containing alloys are relatively more oxidation resistant as compared to the alloy containing Mo3Si because of the superior oxidation resistance of Mo5Si3 phase (Ref 2,3,4). Therefore, constituent phases of the alloy and their fractions are important which decides the ultimate mechanical and oxidation properties of a specific Mo-Si-B-based alloy. Optimization of mechanical properties and high temperature oxidation resistance of Mo-Si-B-based alloys relies on tailoring of microstructure with respect to phase fractions and to some extent by micro-alloying additions (Ref 1, 6,7,8). However, the optimization still faces many challenges because of the pesting phenomenon (catastrophic oxidation) in the intermediate temperatures range (Ref 9,10,11,12,13). Application of oxidation resistant coating over Moss rich, Mo-Si-B or Mo-Ti-Si is another approach for such applications, where mechanical property is important and trading off of mechanical properties is not recommended (Ref 14,15,16,17).
Yang et al. (Ref 6) reported that the macro-alloying of Mo-Si-B alloy with Ti was able to suppress the formation of comparatively oxidation prone Mo3Si phase by replacing with Mo5Si3 (T1 phase). Attempts were made to enhance the oxidation resistance of Mo5Si3, since, Mo5Si3 is not fully protective against oxidation below 1600 °C because of formation of a porous silica layer. The major breakthrough was achieved when researchers found Mo-37Si-40Ti alloy (close to single phase (Mo, Ti)5Si3) to be oxidation resistant in the intermediate temperature range 750-1300 °C (Ref 18,19,20). This approach has opened up a new dimension for study of quaternary Mo-Si-B-Ti alloys. Further studies also corroborated that Mo-Si-B alloys with addition of Ti exhibit superior oxidation resistance when compared to B-free Mo-Ti-Si or Ti-free Mo-Si-B alloys by getting rid of pesting along with good creep resistance and lower density (Ref 21,22,23,24,25,26,27,28,29,30,31). In contrast to the previous approaches of designing Mo-Si-B alloys with maximizing the relatively brittle intermetallic phase, Mo5SiB2 for imparting oxidation resistance by trading off of mechanical properties, especially fracture toughness, the approach of macro-alloying relies on the addition of Ti as the passivating element for achieving optimum microstructure consisting of (Mo, Ti)ss, (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phases. Although the beneficial effect of addition of Ti in Mo-Si-B system is reported but the mechanism is not well understood.
In the present investigation, the microstructure, micro-mechanical properties and oxidation resistance of Mo-35Ti-10Si and Mo-35Ti-10Si-2B (wt.%) alloys were investigated and discussed. We present experimental proof that Mo-35Ti-10Si-2B alloy can be made oxidation resistant by protective multi-layer oxide scales up to 1250 °C. Underlying oxidation mechanism and role of B in improving the oxidation resistance of the alloys is discussed by comparing the oxidation resistance of B free Mo-35Ti-10Si (wt.%) alloy. The micro-mechanical behaviors of three constitutive phases of the alloys were studied by nano-indentation and micropillar compression for investigating the microstructure–property correlation.
2 Experimental Procedures
Mo-35Ti-10Si (wt.%) and Mo-35Ti-10Si-2B (wt.%) alloys were prepared by arc melting of constituent elements Mo, Si, Ti and B, in a Zr-gettering high-purity Ar atmosphere. The alloys were melted multiple times for achieving homogeneity with controlled melting process by optimizing the parameters such as current and vacuum levels to avoid evaporation of alloying elements especially boron and silicon. The microstructure and compositional examinations were carried out using SEM (Model MV2300CT/100, Camscan, UK make) attached with EDS (Model: X-max 80, Oxford make). The microstructures were also observed after the annealing at 1250 °C for 100 h. The phase analysis was carried out by EBSD (Oxford Model: Nordlys Nano) and XRD (Inel-make unit, model MPD).
For generating samples for oxidation studies, multiple coupons of dimensions 5 mm × 3 mm × 3 mm were generated from the arc melted button using EDM wire cutting followed by metallographically polishing of all the sides. Non-isothermal oxidation studies were carried out in an in-house developed thermogravimetry analyzer (TGA) in static air condition with online weight change recording facility. During heating of the furnace, the samples were kept in the cold zone. The coupons were introduced into the hot zone of the furnace for isothermal oxidation tests, only when the temperature reached the desired temperature. The isothermal oxidation studies were carried out at 825, 1000 and 1250 °C for different durations up to 100 h. The oxidized samples were characterized for phase analysis using XRD and the nature of oxide layers.
Since, boron analysis by EDS is not reliable because of low atomic number; therefore, Particle Induced Gamma-ray Emission (PIGE, FOTIA, BARC) (Ref 30) was used for boron analysis. Microhardness of the polished samples were measured using Vicker’s hardness tester with a load of 100 g and a dwell time of 10 s. Hardness and elastic modulus mapping was carried out using a KLA Tencor make nano-indentation (Model: iMicro®)). The detailed methodology was discussed elsewhere (Ref 31, 32).
A number of micropillars were fabricated from three constituent phases [(Mo, Ti)ss, (Ti, Mo)5Si3, and (Mo, Ti)5SiB2] of Mo-35Ti-10Si-2B (wt.%) using a dual beam focused ion beam (FIB) in a SEM (Carl Zeiss FESEM). EDS and EBSD analysis were carried out to identify the phases before fabrication of the micropillars. Those grains with suitable orientations were selected for fabricating micropillar which possesses non-zero (preferably > 0.3) Schmid factor for at least one possible slip system. The sizes of micropillars were decided based on the size of the respective phases to avoid presence of other phases in a specific micropillar. Initial milling was done using Ga + ions beam with a current of 16 nA for fabricating a circular trench with the outer diameter of 40 µm and a coarse pillar of diameter of around 10 µm at the center. Subsequently, the current was gradually lowered to 0.1 nA to fabricate polished micropillars with a final nominal top diameter of 1.5-5 µm and aspect ratio (L/D) of ~ 2.5. Compression experiments using diamond flat punch were conducted using a nano-indenter in displacement-controlled mode at room temperature as described elsewhere (Ref 33). The compression tests on all the micropillars were carried out at a strain rate of 0.05/s. The deformed micropillars were observed under SEM for understanding their deformation behavior.
3 Results and Discussion
3.1 Microstructural Characterization
Figure 1(a) and (b) shows the representative back scattered electron (BSE) images of Mo-35Ti-10Si and Mo-35Ti-10Si-2B (wt.%) alloys, respectively. Mo-35Ti-10Si alloy was found to be consisting of two phases; while, Mo-35Ti-10Si-2B alloy was found to be comprised of three phases as seen in the BSE SEM images. EDS point analyses were carried out in different phases of the alloys and the results are summarized in Table 1.
From the EDS point analysis and XRD analysis, the Mo-35Ti-10Si alloy was found to be consisting of (Mo, Ti)ss, and (Mo, Ti)3Si phases. The detailed microstructural characterization was carried out for the Mo-Ti-Si alloys system in our previous study (Ref 30, 31). The EBSD band contrast image, phase map and inverse pole figures obtained from the Mo-35Ti-10Si-2B alloy is shown in Fig. 2. By comparing the EDS point analysis data, and EBSD analysis, constitutive phases were confirmed as (Mo, Ti)ss, (Mo, Ti)5SiB2 and (Ti, Mo)5Si3. XRD patterns shown in Fig. 3 confirmed the presence of the identified phases.
External (in air) Particle Induced Gamma-ray Emission (PIGE) facility at FOTIA, BARC, was used for non-destructive quantification of Ti, Si and B in Mo-35Ti-10Si-2B alloy. Direct samples were placed outside the beam exit window in the external PIGE set up. The samples were irradiated using 3.5 MeV proton beam of ~ 10nA current. 429, 718 and 2125 keV prompt gamma rays from 10B(p,αγ)7Be, 10B(p,p′γ)10B and 11B(p,p′γ)11B, 983 keV of 48Ti(p,p′γ)48Ti and 1273 and 1779 keV of 29Si(p,p′γ)29Si and 28Si(p,p′γ)28Si were detected during irradiation using HPGe detector for quantification of B, Ti and Si in the alloy. PIGE spectrum is shown in Fig. 4 with various peaks of different isotopes of B, Ti and Si. The boron loading in the alloy was found to be 1.983 ± 0.056 determined through PIGE analysis, which confirmed negligible evaporation losses of boron during melting multiple times.
3.2 Oxidation Behavior
3.2.1 Effect of Boron on Oxidation Behavior at 825 °C
Figure 5 shows the TGA plots for oxidation during continuous heating followed by isothermal holding of 1 h at 825 °C of the two alloys. From the TGA plots, it can be seen that initially there was no weight loss up to 800 °C followed by weight loss per unit surface area of about 25 mg/cm2 and about 0.025 mg/cm2 for Mo-35Ti-10Si and Mo-35Ti-10Si-2B alloys, respectively. The large difference of three orders in the weight loss per unit surface area is because of the continuous mass loss from the Mo-35Ti-10Si in the form of volatile MoO3. The main reason of the insufficient protectiveness of the oxide scale formed on Mo-35Ti-10Si alloys is rapid inward transport of oxygen through channels and pores in the scale. It has previously been reported that pesting resistance in Mo-Si-Ti alloys depends mainly on the Ti content within the phases of the respective alloy (Ref 25,26,27,28). On the other hand, Mo-35Ti-10Si-2B alloy showed excellent oxidation resistant suggesting substantial effect of B in enhancing the oxidation resistance of the alloy.
Figure 6(a) and (b) shows the BSE SEM image showing the microstructures of the top surfaces (as-oxidized) after oxidation at 825 °C in non-isothermal condition for Mo-35Ti-10Si and Mo-35Ti-10Si-2B alloys, respectively. Large pores and voids are visible in the microstructure of the oxidized Mo-35Ti-10Si alloy; whereas, impervious oxide layer was found to develop over Mo-35Ti-10Si-2B alloy. This is the reason of the observation of lower mass loss during the oxidation at 825 °C as shown in Fig. 5. The oxide scale of the Mo-35Ti-10Si alloy was found to be consisting of SiO2-TiO2 with pores created due to the volatization of MoO3. To overcome the relatively poor oxidation behavior of porous SiO2-TiO2 outer oxide layer because of the faster ingress of oxygen, there must be some mechanism to make the layer impervious by decreasing the viscosity of the protective oxide by forming glassy phase whose diffusivity is lower for O2− diffusion. It is well-known that addition of B reduces the viscosity of silica by forming borosilica SiO2-B2O3 significantly, which helps in fast sealing of the substrate by covering the pores immediately when it forms (due to the evaporation of MoO3) resulting in formation of protective oxide layer. To understand the oxidation mechanism and development of nature of oxide layer, the Mo-35Ti-10Si-2B alloy was subjected to isothermal oxidation in the temperature regime of 825-1250 °C for different durations.
3.2.2 Isothermal oxidation of Mo-35Ti-10Si-2B alloy in the temperature range of 825-1250 °C
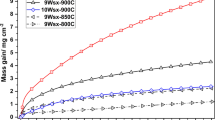
Figure 7 shows the change in weight during oxidation per unit surface area with respect to time for Mo-35Ti-10Si-2B alloy obtained during the isothermal oxidation tests at three different temperatures between 825 and 1250 °C in static air condition. Steady state was found to reach after initial mass gain in the temperatures range from 1000 to 1250 °C and initial mass loss for 825 °C, demonstrating a significantly enhanced oxidation resistance. The appearances of the specimens after oxidation at different temperature and time were observed and found that the samples were intact even after oxidation at 1250 °C for 100 h, unlike the conventional Mo based alloy which disintegrate at such oxidation conditions. The color (appearance) of the oxidized samples was found to change from gray to yellowish gray from temperature 825-1250 °C.
Figure 8 presents the BSE SEM image showing the microstructure of the alloy and corresponding microstructures of the top surfaces (as-oxidized) after oxidation at 825, 1000 and1250 °C for 100 h. The higher magnification images are also shown in the respective insets. All the surfaces were mostly found crack free. Striking differences can be observed for the oxide morphologies at different temperatures. Substantial increase in oxide formation can be seen with increase in temperature. Duplex, SiO2-TiO2 oxide layer was observed for the surfaces oxidized at 825 and 1000 °C; whereas, dense TiO2 oxide layer with tightly packed TiO2 crystals were found to be developed for the surface oxidized at 1250 °C.The morphological evidence of elongated (Mo, Ti)5SiB2 phase can be seen intact even after 100 h of oxidation showing the excellent oxidation stability. After evaporation of MoO3 from (Mo, Ti)ss phase, the oxide layer become porous but because of the formation of borosilica and TiO2 discontinuous crystals the oxide layer shows oxidation resistance, as the time progresses.
Figure 9(a) shows the cross section of the alloy oxidized at 825 °C for 100 h showing the increase in thickness of oxide scale with temperature. Underneath the duplex oxide layer, an internal oxidation zone was found to develop consisting of mixed oxides of Mo, Si and Ti for the sample oxidized at 825 °C.
As the temperature of oxidation increases, a tendency to form a pure TiO2 outer layer was observed and no internal oxidation zone were observed. Figure 8(b) shows the cross section image of the oxidized sample at 1250 °C for 100 h revealing the multi-layer characteristics of the oxide structure. The oxide structure was found to be consisting of distinct layer of TiO2 (outer most) followed by duplex oxide layer in the oxide-substrate side. Mitchell et al. (Ref 34) observed that based on the anionic volume ratio the protectiveness of the oxide layer can be predicted and based on the analysis the outermost TiO2 layer is considered to be a dense and protective layer. The duplex layer was found to be consisting of SiO2 base amorphous phase and discrete phase of TiO2. Si, O, and small amounts of B was detected through EDS point analysis in the amorphous region with stoichiometric chemistry of SiO2. The low magnification cross section analysis was carried out to study the contribution of different constituent phases on the oxidation resistance. Figure 10(a) shows the clear presence of outer TiO2 layer and inner borosilica layer containing TiO2 particles. Oxidation products could be seen propagated through (Mo, Ti)ss, (shown in the encircled region in Fig. 10a) whereas (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phases found to act as the oxidation resistant barriers against oxygen attack during the oxidation. The free energy of formation of all the probable oxides at 1250 °C were calculated using the PO2 level for the internal area and found to be B2O3 (− 505.737 kJ/mol) > TiO2 (− 377.25 kJ/mol) > SiO2 (− 282.59 kJ/mol) > MoO3 (− 249.524 kJ/mol). From the free energy of formation of oxides, this can be seen that the probability of formation of B2O3 as the first layer is high; however, it also has the tendency to vaporize at higher temperature as the vapor pressure of B2O3 and other sub-oxides of boron are quite high. On the other hand, the as per the free energy of formation of oxides, the next probable oxide to form is TiO2. The vapor pressure of TiO2 at 1250 °C is 2.007 × 10−12 atm which is very less and expected vaporization loss is extremely low. This is the reason why TiO2 was also observed experimentally to be the outermost oxide layer. As TiO2 is an oxygen conductor, it allows oxygen to ingress in controlled way which felicitate the formation of SiO2 and B2O3 followed by formation of protective borosilica underneath the outer layer. (Mo, Ti)5SiB2 phase was observed to be the most oxidation resistant among the three phases and the responsible phase for imparting the improved oxidation resistance to the alloy through the oxidation product B2O3.
Figure 11 shows the EBSD maps of the duplex SiO2-TiO2 layer (marked in Fig. 10b) formed at 1250 °C, which indicates the presence of crystalline rutile and amorphous borosilica phase. The volume fractions of titania (TiO2) and borosilica (SiO2-B2O3) are about 43 and 57%, respectively, determined from the phase analysis through EBSD and confirmed by image analysis of SEM BSE image. The formation of amorphous phase was also confirmed by the XRD analysis. Figure 12 shows the XRD pattern which indicates presence of hump which is a typical characteristic of an amorphous phase.
Schliephake et al. (Ref 29) studied oxidation behavior of Mo-9Si-8B-29Ti (in at.%) alloy and they found the oxide layer to be consisting of TiO2 as the outermost layer at the oxide–air interface followed by a thick and porous duplex layer, comprising SiO2 and TiO2 in the substrate–oxide interface. Because of the microporous duplex layer, the alloy showed limited oxidation resistance. However, the current alloy (Mo-35Ti-10Si-2B) exhibited superior oxidation resistance without showing any porous oxide layer formation. The oxidation process at higher temperatures (1000-1250 °C) proceeds by formation of an impervious outer TiO2 layer followed by development of amorphous SiO2-B2O3 layer. Although, TiO2 is a good conductor of O2− ion, the formation of the inner impervious borosilica prevents the further oxidation of the alloy substrate. Also, the presence of the impervious (about 4 µm after 100 h at 1250°) and continuous outer TiO2 layer hinders the evaporation loss of B2O3 keeping the glassy borosilica phase intact. Hence, the excellent oxidation behavior of the investigated alloy has been detected in 1000-1250 °C.
3.3 Micro-mechanical Behavior
3.3.1 Hardness and Elastic Modulus
The microhardness was determined by Vickers microhardness using a load of 100 g. The microhardness of the Mo-35Ti-10Si-2B alloy was found to be 1210 ± 35 HV from randomly selected 10 positions. The mechanical properties of individual phases were determined by nano-indentation technique in depth-controlled mode. Figure 13 presents the representative load versus indentation depth plots determined for constituent phases [(Mo, Ti)ss, (Ti, Mo)5Si3, and (Mo, Ti)5SiB2] of Mo-35Ti-10Si-2B alloy. The plots clearly reveal the different peak loads, Pmax (which are used for indentation measurements) for three phases. (Mo, Ti)ss phase was found to be softer and therefore peak load was the least followed by (Ti, Mo)5Si3 and (Mo, Ti)5SiB2 at a fixed depth test mode. The load versus indentation depth plots were analyzed by the methodology proposed by Oliver–Pharr (Ref 35,36,37,38) for determining the hardness and elastic modulus. The hardness were found to be in the range of 8.5, 15, and 25 GPa for (Mo, Ti)ss, (Ti, Mo)5Si3, and (Mo, Ti)5SiB2 phases, respectively. The elastic modulus were found to be in the range of 250, 375, 460GPa for (Mo, Ti)ss, (Ti, Mo)5Si3, and (Mo, Ti)5SiB2 phases, respectively. The values of hardness and elastic modulus of (Mo, Ti)ss, (Ti, Mo)5Si3, and (Mo, Ti)5SiB2 are not available in the literature; however, the hardness values reported for (Mo, Ti)ss, Mo5Si3, and Mo5SiB2 phases are 7.7 (Ref 4), 11.8 (Ref 39), 20.8 GPa (Ref 40), respectively are varying slightly from the reported values of their counterparts because of the presence of Ti in their lattices. Such variations arise if the indentation loads are different causing different indentation size and referred as the indentation size effect (Ref 34). The ratios H3/E2 for each phase were also calculated (tabulated in Table 2), which is believed to be an effective parameter to determine the resistance against the plastic deformation (Ref 34). Both the intermetallic phases, (Ti, Mo)5Si3, and (Mo, Ti)5SiB2show a higher H3/E2 values, indicating the limited capabilities of plastic deformation because of the much high hardness.
A high-speed nano-indentation mapping technique was utilized to determine the hardness and elastic modulus of the constituent phases of (Mo, Ti)ss, (Mo, Ti)5SiB2, and (Ti, Mo)5Si3. Diamond Berkovich tip with a Young’s modulus of 1141 GPa and a Poisson’s ratio of 0.07 was used for this purpose. The results of the mapping are presented in Fig. 13. With the advanced mapping protocol, a single indent can be completed in under one second, which encompasses the duration required for surface approach, surface detection, loading, unloading, and relocating the sample for the subsequent indentation in the array (Ref 37). Since the possibility of fast indentation speed was made available by decreasing the gap between two consecutive indents in the array, high-resolution mapping within the micrometer range using hundreds of indents is possible to carry out in reasonable time frame. This technological advancement opens up the possibility of increasing the number of indents enhances the resolution of spatial mapping. Using a grid pattern, indentations were performed on Mo-35Ti-10Si-2B alloy with varying loads ranging from 10 to 50 mN and at indentation intervals between 4 and 10 µm. The indentation load was selected in such a way to create a gap of spacing to indent depth ratio of ~ 10 (Ref 37). The hardness as well as elastic modulus of all the indents were determined using a method proposed by Oliver and Pharr method (Ref 35, 36). The distribution of the distinct constitutive phases was mapped and identified on the basis of hardness and elastic modulus of the respective phases. Figure 14(a) presents an optical micrograph of the indentation arrays and corresponding maps of hardness and elastic modulus illustrating a one-to-one correlation with the horizontally flipped optical image.
3.3.2 Micropillar Compression Behavior of Individual Phases
Micropillar compression technique was adopted to investigate the mechanical properties of the constitutive phases of Mo-35Ti-10Si-2B alloy. This technique was first introduced by Uchic et al. (Ref 41), which is now identified as a new tool to examine the micro-mechanical responses of different materials including pure metals, alloys and intermetallics at size scale of less than 10 µm (Ref 42,43,44,45,46,47).
Figure 15(a), (b) and (c) shows the SEM images of the micropillars fabricated from (Mo, Ti)5SiB2, (Ti, Mo)5Si3 and (Mo, Ti)ss phases, respectively, revealing the outlook of the as fabricated micropillars. The images shown in the inset shows the images observed along the direction inclined by 30° from the loading axis. Figure 15(d), (e) and (f) shows the representative stress–strain curves obtained from the micro-compression testing of micropillar specimens prepared in different phases of (Mo, Ti)ss, (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 (shown in Fig. 14a, b and c). The diameter of the micropillars were 3.5, 7 and 6 µm for (Mo, Ti)ss, (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phase, respectively, which was decided by the microstructural size range of the respective phases. The aspect ratio was kept similar (~ 2.5) for all the fabricated micropillars. Attempts were made to stop the compression tests before failure occurs for the ease of observations of deformation behavior by examining slip lines. (Mo, Ti)5SiB2phase exhibited the highest strength (5.9 GPa) followed by (Ti, Mo)5Si3(4.5 GPa) and then (Mo, Ti)ss (2.8 GPa). Nano-indentation studies have also shown that the highest hardness is exhibited by the (Mo, Ti)5SiB2phase followed by the (Ti, Mo)5Si3 phase and then the (Mo, Ti)ss which agrees qualitatively with the observed trend of compressive strength (Ref 28, 29).
(a–c) shows the SEM images of the micropillars fabricated in (Mo, Ti)5SiB2, (Ti, Mo)5Si3 and (Mo, Ti)ss phases, respectively. Inset shows the images observed along the direction inclined by 30ofrom the loading axis. (d–f) Show the stress–strain curves obtained from the micro-compression testing of micropillar specimens prepared in different phases. (g–i) Shows SEM images of corresponding deformed micropillars.
On analyzing the stress–strain curves, it was found that all the phases showed a significant amount of deformation before failure in micron size range. Micropillars of (Mo, Ti)ss phase showed the maximum plastic strain before the test was stopped. The values of elastic modulus determined from the stress–strain curves were found identical with the literature values (averaged value of Mo and Ti) and the values obtained from the nano-indentation of the individual phases. Figure 15(g), (h), (i) shows SEM images of corresponding deformed micropillars. (Mo, Ti)ss phase revealed ductile behavior which was also confirmed by the observed dislocation activities corroborated by faint slip line observed in fractography (Fig. 15i). Formation of slip lines (shown by arrows) was seen in the deformed micropillar as shown in Fig. 15(i). On the other hand, the micropillars (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phase failed at a stress level of 5.9 and 5.5GPa, respectively, exhibiting only small plastic strain prior to failure (Fig. 15g and h) before the compression test could be stopped. Significant amount of strain bursts was found as the flat portions of the stress–strain curves (Fig. 15d and e) just after yielding for micropillars for (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phases which is the indication of activation of dislocation activities. Strain bursts are generally found to occur because of the avalanche like collection of dislocation motions during compression tests of micron sized specimens (Ref 48, 49). Since, the strain bursts after the elastic region corresponds to the formation of a slip bands due to yielding, the motion of dislocations in brittle (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phases may not be so difficult and can also be activate at room temperature. These indicate that the room temperature fracture toughness of the alloy can be adequate by proper microstructural tailoring, which would be an interesting research area.
Ti5Si3 has a hexagonal D88 structure (Ref 43). Kishida et al. (Ref 43) carried out micropillar testing of Ti5Si3 and reported that plastic flow at room temperature was possible when small single crystals of the micron-meter size were tested in compression. Small amount of plasticity is shown by (Ti, Mo)5Si3 in the present study also, and the evidence for the same can be obtained from H3/E2 ratio. The value of H3/E2 was found to be 0.024 for (Ti, Mo)5Si3, which is one order lower than the value of 0.123 reported for silicide phase, Mo3Si (Ref 4).
Mo5SiB2 has a complex body centered Cr5B3 type tetragonal structure (Ref 44). The crystal structure is layered along the tetragonal c-axis and the plastic flow has been reported to be very difficult below 1500 °C even at low strain rates. However, the deformability of Mo5SiB2 was shown by Kishida et al. (Ref 44) in the micropillar form, unlike in the bulk form. (Mo, Ti)5SiB2 was found to be fairly deformable as shown in the stress–strain diagram (Fig. 6f). The primary feature of the stress–strain curve was found to be a large strain burst followed by fracture. The sudden failure of the pillar is probably because of the localization of strain. Similar behavior has been reported by Song et al. (Ref 50) for a quasi-crystalline approximant where there is strong evidence that strain localization did not proceed via dislocation movement (Ref 50). The value of H3/E2 was found to be 0.073 for (Mo, Ti)5SiB2, which is slightly lower than the value of 0.077 (Ref 33) reported for Mo5SiB2 indicating role of Ti in improving deformability. The effect of addition of Ti on mechanical properties of Mo Alloys were reported elsewhere (Ref 25, 28, 29). Higher critical resolved shear stress (CRSS) than fracture stress for a slip system at room temperature results in brittleness of an alloy or in articular a phase, causing difficulty in dislocation motion. Kishida et al. reported CRSS for Mo silicides as well as Ti5Si3 by micropillar compression testing at room temperature for a wide range of orientations (Ref 43, 51). The role of Ti in decreasing the CRSS of (Mo, Ti)5Si3 and (Mo, Ti)5SiB2 should be studied in detail in future to conclude anything with confidence.
4 Conclusion
The effect of microstructural constituting phases on the intermediate to high-temperature oxidation behavior of Mo-35Ti-10Si and Mo-35Ti-10Si-2B (wt.%) alloys were evaluated. SEM, EDS, EBSD and PIGE analysis revealed the constitutive phases as (Mo, Ti)ss, (Mo, Ti)3Si and (Mo, Ti)ss, (Ti, Mo)5Si3 and (Mo, Ti)5SiB2 for the Mo-35Ti-10Si and Mo-35Ti-10Si-2B alloy, respectively. Mo-35Ti-10Si alloys was not found to be fully protective against oxidation because of the microporous oxide layer; whereas, Mo-35Ti-10Si-2B alloy exhibited superior oxidation resistance with very small mass loss (about 0.025 mg/cm2) in the intermediate temperature of oxidation at 825 °C. Fast and complete passivation of Mo-35Ti-10Si-2B was possible in the temperature range of 1000-1250 °C. Cross sectional analysis of the oxidized Mo-35Ti-10Si-2B alloy sample revealed the formation of duplex oxide layer consisting of SiO2-TiO2 with internal oxidation zone at the intermediate temperatures of 825 °C. On the other hand, formation of the protective borosilica and double oxide layers (TiO2 and duplex borosilica-TiO2) was revealed after 100 hours of oxidation at 1250 °C. The oxide composition, morphology of the duplex layer and compactness of the outermost rutile layer was found to play a vital role on the oxidation resistance of Mo-Si-B-Ti alloys. It was found that the oxide scale with a continuous silica (amorphous/glassy) matrix and embedded TiO2 (rutile) phase provides excellent barrier against oxidation in the temperature range of 825-1250 °C for Mo-Si-Ti-B alloy system, wherein B helps in facilitating formation of amorphous/glassy duplex layer.
The micro-mechanical behaviors of three constitutive phases of the alloys were studied by nano-indentation and micropillar compression. The mechanical properties of the three constituent phases were experimentally measured and the fracture stress, hardness and elastic modulus were found to be (3, 6 and 5GPa); (8.5, 15, and 25 GPa) and (250, 375, 460GPa), for (Mo, Ti)ss, (Ti, Mo)5Si3, and (Mo, Ti)5SiB2 phases, respectively. We report the stress–strain behavior of the constituent phases of Mo-35Ti-10Si-2B (wt.%) alloy by micropillar compression for the first time. The stress–strain behavior showed that (Mo, Ti)5SiB2 and (Ti, Mo)5Si3 phases exhibited a cleavage failure after a significant strain burst, whereas (Mo, Ti)ss phase has shown ductile behavior which was also confirmed by the observed dislocation activities in the form of slip lines.
References
T.M. Pollock, Alloy Design for Aircraft Engines, Nat. Mater., 2016, 15, p 809–815.
J. Wang, X. Zhang, G. Zhang, B. Li, R. Li, and S. Li, Effect of Si Solution in α-Mo on Oxidation Behavior of Mo-Si-B Alloy and Oxidation Kinetics Model, Int. J. Refract Metal Hard Mater., 2023, 116, 106325.
S. Majumdar, Isothermal and Cyclic Oxidation Resistance of Pack Siliconized Mo–Si–B Alloy, Appl. Surf. Sci., 2017, 414, p 18–24.
Y. Liu, Y. Li, H. Kou, Y. Chen, F. Zhang, and X. Song, Microstructural and Hardness Investigation of a Multiphase Mo-Si-B Alloy Processed by Laser Surface Remelting, Surf. Coat. Technol., 2022, 450, p 129012.
Mi. Zhao, Xu. Boyang, Y. Shao, Y. Zhu, Wu. Jie, Wu. Shusen, and Y. Yan, Microstructure and Oxidation Mechanism of Multiphase Mo–Ti–Si–B Alloys at 800 °C, Corros. Sci.. Sci., 2021, 187(15), p 109518.
S.K. Makineni, A.R. Kini, E.A. Jägle, H. Springer, D. Raabe, and B. Gault, Synthesis and Stabilization of a New Phase Regime in a Mo-Si-B Based Alloy by Laser-Based Additive Manufacturing, Acta Mater. Mater., 2018, 151, p 31–40.
J. Becker, S. Schmigalla, S. Schultze, S.-K. Rittinghaus, A. Weisheit, J. Schmelzer, and M. Krüger, High Temperature Oxidation Performance of an Additively Manufactured Mo–9Si–8B Alloy, Oxid. Met.. Met., 2022, 97, p 167–181.
K. Pan, Y. Yang, S. Wei, H. Wu, Z. Dong, Y. Wu, S. Wang, L. Zhang, J. Lin, and X. Mao, Oxidation Behavior of Mo-Si-B Alloys at Medium-to-High Temperatures, J. Mater. Sci. Technol., 2021, 60, p 113–127.
M. Jin, D. He, W. Shao, Z. Tan, X. Guo, Z. Zhou, XuWu. Guohong Wang, Li. Cui, and L. Zhou, Influence of B Contents on the Microstructure, Fracture Toughness and Oxidation Resistance of Mo-Si-B Alloys, J. Alloy. Compd., 2022, 890(15), p 161829.
K. Pan, Y. Yang, S. Wei, H. Wu, Z. Dong, Y. Wu, S. Wang, L. Zhang, J. Lin, and X. Mao, Oxidation Behavior of Mo–Si–B Alloys at Medium-to-High Temperatures, J. Mater. Sci. Technol., 2021, 60, p 113–127.
B. Paul, S. Koley, and A.K. Suri, A Novel Approach to Determine Oxidation Kinetics of Mo–16Cr–xSi(x = 4–6 wt.%) Alloy Using Stepwise Isothermal Thermo-Gravimetry, Thermochim. Acta. Acta, 2012, 549, p 57–62.
B. Paul, S. Majumdar, and A.K. Suri, Microstructure and Mechanical Properties of Hot Pressed Mo–Cr–Si–Ti In-Situ Composite, and Oxidation Behavior with Silicide Coatings, Int. J. Refract. Met. Hard Mater., 2013, 38, p 26–34.
Y. Yang, Y.A. Chang, L. Tan, and W. Cao, Multiphase Equilibria in the Metal-Rich Region of the Mo–Ti–Si–B System: Thermodynamic Prediction and Experimental Validation, Acta Mater. Mater., 2005, 53, p 1711–1720.
C. Gatzen, I. Smokovych, M. Scheffler, and M. Kruger, Oxidation-Resistant Environmental Barrier Coatings for Mo-Based Alloys: A Review, Adv. Eng. Mater., 2021, 23, p 2001016.
W. Yang, K. Choi, K.H. Baik, Y. Kim, S. Lee, J. Kim, J. Lee, and J.S. Park, Oxidation Behaviors of Si/Al Pack Cementation Coated Mo-3Si-1B Alloys at Various Temperatures, Met. Mater. Int., 2021, 27, p 914–921.
B. Paul, P.K. Limaye, R.C. Hubli, and A.K. Suri, Microstructure and Wear Properties of Silicide-Based Coatings over Mo–30W Alloy, Int. J. Refract. Met. Hard Mater., 2014, 44, p 77–83.
B. Paul, S.P. Chakraborty, and A.K. Suri, Formation of Silicide Based Oxidation Resistant Coating Over Mo-30 wt% W ALLOY, Trans. Indian Ceram. Soc., 2013, 72, p 39–42.
M. Azim, S. Burk, B. Gorr, H.-J. Christ, D. Schliephake, M. Heilmaier, R. Bornemann, and P.H. Bolivar, Effect of Ti (Macro)Alloying on the High-Temperature Oxidation Behavior of Ternary Mo-Si-B Alloys at 820–1300°C, Oxid. Met., 2013, 80, p 231–242.
M.A. Azim, B. Gorr, H.-J. Christ, O. Lenchuk, K. Albe, D. Schliephake, and M. Heilmaier, Effect of Ti Content and Nitrogen on the High-Temperature Oxidation Behavior of (Mo, Ti)5Si3, Intermetallics, 2017, 90, p 103–112.
M.A. Azim, D. Schliephake, C. Hochmuth, B. Gorr, H.-J. Christ, U. Glatzel, and M. Heilmaier, Creep Resistance and Oxidation Behavior of Novel Mo-Si-B-Ti Alloys, JOM, 2015, 67, p 2621–2628.
D. Schliephake, M. Azim, K.V. Klinski-Wetzel, B. Gorr, H.-J. Christ, H. Bei, E.P. George, and M. Heilmaier, High-Temperature Creep and Oxidation Behavior of Mo–Si–B Alloys with High Ti Contents, Metall. Mater. Trans., 2014, 45(45), p 1102–1111.
S. Majumdar, B. Paul, P.K. Singh, J. Kishor, and V. Kain, Effect of Si Content on Microstructure, Mechanical and Oxidation Properties of Hot-Pressed Mo-Ti-Si Alloys, Intermetallics, 2018, 100, p 126–135.
B. Paul, A. Srakar, J. Kishor, and S. Majumdar, Studies on Creep and Wear Behavior of Mo-40Ti-10Si Alloy Prepared by Hot Pressing, J. Mater. Eng. Perform., 2020, 29, p 1007–1014.
D. Schliephake, A. Kauffmann, X. Cong, C. Gombola, M. Azim, B. Gorr, H. Christ, and M. Heilmaier, Constitution, Oxidation and Creep of Eutectic and Eutectoid Mo-Si-Ti Alloys, Intermetallics, 2019, 104, p 133–142.
S. Obert, A. Kauffmann, and M. Heilmaier, Characterisation of the Oxidation and Creep Behaviour of Novel Mo-Si-Ti Alloys, Acta Mater. Mater., 2020, 184, p 132–142.
S. Obert, A. Kauffmann, S. Seils, S. Schellert, M. Weber, B. Gorr, H. Christ, and M. Heilmaier, On the Chemical and Microstructural Requirements for the Pesting-Resistance of Mo–Si–Ti Alloys, J. Mater. Res. Technol., 2020, 9, p 8556–8567.
S. Obert, A. Kauffmann, S. Seils, T. Boll, S. Weiss, H. Chen, R. Anton, and M. Heilmaier, Microstructural and Chemical Constitution of the Oxide Scale Formed on a Pesting-Resistant Mo-Si-Ti Alloy, Corros. Sci.. Sci., 2021, 178, p 109081.
S. Obert, A. Kauffmann, R. Pretzler, D. Schliephake, F. Hinrichs, and M. Heilmaier, The Creep and Oxidation Behaviour of Pesting-Resistant (Mo, Ti)5Si3-Containing Eutectic-Eutectoid Mo-Si-Ti Alloys, Metals, 2021, 11, p 169.
A.S. Tirunilai, F. Hinrichs, D. Schliephake, M. Engstler, F. Mücklich, S. Obert, G. Winkens, A. Kauffmann, and M. Heilmaier, Phase Continuity, Brittle to Ductile Transition Temperature, and Creep Behavior of a Eutectic Mo–20Si–52.8Ti Alloy, Adv. Eng. Mater., 2022, 24, p 2200918.
R. Acharya, S.K. Wasim Raja, S. Chhillar, J. Gupta, J.K. Sonber, T.S.R.C. Murthy, K. SasiBhushan, R.M. Rao, S. Majumdar, and P.K. Pujari, Non-destructive quantification of total boron and its isotopic composition in boron based refractory materials by PIGE and an inter-comparison study using TIMS and titrimetry, J. Anal. At. Spectrumetry, 2018, 33, p 784.
P. Suresh Babu, P. Chanikya Rao, A. Jyothirmayi, P. SudharshanPhani, L. Rama Krishna, and D. Srinivasa Rao, Evaluation of Microstructure, Property and Performance of Detonation Sprayed WC-(W, Cr)2C-Ni Coatings, Surf. Coat. Technol., 2018, 335, p 345.
B. Paul, J. Kishor, A. Karthik, K. Singh, and S. Majumdar, Studies on the Tribological Performance, Characterization and Mechanical Properties of W-2Ni-1Fe (wt%) Alloy, Int. J. Refract. Met. Hard Mater., 2022, 103, p 105752.
Z. Chen, B. Paul, S. Majumdar, H. Inui, and S. Otani, Room-Temperature Deformation of Single Crystals of ZrB2 and TiB2 with the Hexagonal AlB2 Structure Investigated by Micropillar Compression, Sci. Rep., 2021, 11(1), p 14265.
T.E. Mitchell, D.A. Voss, and E.P. Butler, The Observation of Stress Effects during the High Temperature Oxidation of Iron, J. Mater. Sci., 1982, 17, p 1825–1833.
W.C. Oliver and G.M. Pharr, An Improved Technique for Determining Hardness and Elastic Modulus Using Load Displacement Sensing Experiments, J. Mater. Res., 1992, 7, p 1564–1583.
W.C. Oliver and G.M. Pharr, Measurement of Hardness and Elastic Modulus by Instrumented Indentation: Advances in Understanding and Refinements to Methodology, Mater. Res. Soc., 2004, 9, p 3–20.
P. SudharshanPhani and W.C. Oliver, A Critical Assessment of the Effect of Indentation Spacing on the Measurement of Hardness and Modulus Using Instrumented Indentation Testing, Mater. Des., 2019, 164, p 107563.
B. Vignesh, W.C. Oliver, G. Siva Kumar, and P.S. Phani, Critical Assessment of High Speed Nanoindentation Mapping Technique and Data Deconvolution on Thermal Barrier Coatings, Mater. Des., 2019, 181, p 108084.
F. Chu, D.J. Thoma, K. McClellan, P. Peralta, and Y. He, Synthesis and Properties of Mo5Si3 Single Crystals, Intermetallics, 1999, 7, p 611–620.
K. Ihara, K. Ito, and M. Yamaguchi, Mechanical Properties of Mo5SiB2 Single Crystals, Mater. Sci. Eng. A, 2002, 329, p 222–227.
M.D. Uchic, D.M. Dimiduk, J.N. Florando, and W.D. Nix, Sample Dimensions Influence Strength and Crystal Plasticity, Science, 2004, 305, p 986–989.
A.S. Schneider, D. Kaufmann, B.G. Clark, C.P. Frick, P.A. Gruber, R. M€onig, O. Kraft, and E. Arzt, Correlation Between Critical Temperature and Strength of Small-Scale bcc Pillars, Phys. Rev. Lett., 2009, 103, p 105501.
K. Kishida, T. Fukuyama, T. Maruyama, and H. Inui, Room Temperature Deformation of Single Crystals of Ti5Si3 with the Hexagonal D88 Structure Investigated by Micropillar Compression Tests, Sci. Rep., 2020, 10, p 17983.
K. Kishida, T. Maruyama, H. Matsunoshita, T. Fukuyama, and H. Inui, Micropillar Compression Deformation of Single Crystals of Mo5SiB2 with the Tetragonal D8l Structure, Acta Mater. Mater., 2018, 159, p 416–428.
Z.M.T. Chen, N.L. Okamoto, M. Demura, and H. Inui, Micropillar Compression Deformation of Single Crystals of Co3(Al, W) with the L12 Structure, Scr. Mater., 2016, 121, p 28–31.
N.L. Okamoto, S. Fujimoto, Y. Kambara, M. Kawamura, Z.M.T. Chen, H. Matsunoshita, K. Tanaka, H. Inui, and E.P. George, Size Effect, Critical Resolved Shear Stress, Stacking Fault Energy, and Solid Solution Strengthening in the CrFeMnCoNi High-Entropy Alloy, Sci. Rep., 2016, 6, p 35863.
J. Zhang, K. Kishida, and H. Inui, Specimen Size and Shape Dependent Yield Strength in Micropillar Compression Deformation of Mo Single Crystals, Int. J. Plast., 2017, 92, p 45–56.
D.M. Dimiduk, M.D. Uchic, and T.A. Parthasarathy, Size-Affected Single-Slip Behavior of Pure Nickel Microcrystals, Acta Mater. Mater., 2005, 53, p 4065–4077.
B. Devincre, and L. Kubin, Scale Transitions in Crystal Plasticity by Dislocation Dynamics Simulations, C. R. Phys., 2010, 11, p 274–284.
G. Song, T. Kong, K.J. Dusoe, P.C. Canfield, and S.-W. Lee, Shear Localization and Size-Dependent Strength of YCd 6 Quasicrystal Approximant at the Micrometer Length Scale, J. Mater. Sci., 2018, 53, p 6980–6990.
K. Kishida, Z. Chen, H. Matsunoshita, T. Maruyama, T. Fukuyama, Y. Sasai, H. Inui, and M. Heilmaier, Plastic Deformation of Bulk and Micropillar Single Crystals of Mo5Si3 with the Tetragonal D8m Structure, Int. J. Plast., 2022, 155, p 103339.
Acknowledgment
Authors wish to extend sincere thanks to K V Mani Krishna and Nachiket A. Keskar, MMD, BARC for extending their help in FIB milling and imaging supports. Authors wish to thank Dr. Raghunath Acharya, RCD, BARC, Dr. Jyoti Prakash, GAMD, BARC and Dr. Vivekanand Dubey, MP&CED, BARC for arranging the PIGE, Raman spectroscopy and GDOE facilities. The experimental support by Mr. S. K. Gavai, Mr. S. S. Molke and Mr. V. Ramdas of MP&CED, BARC is gratefully acknowledged. The authors also wish to thank Dr. A. Awasthi, Head, MP&CED and Dr. R. Tewari, Director, MG, BARC for their constant encouragement during the course of this work.
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paul, B., Kumar, S., Kishor, J. et al. Effect of Microstructure on Oxidation and Micro-mechanical Behavior of Arc Consolidated Mo-Ti-Si-(B) Alloys. J. of Materi Eng and Perform (2024). https://doi.org/10.1007/s11665-024-09792-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-024-09792-x