Abstract
In order to enhance the durability of tribological interfaces, an investigation into the synergistic effects of sliding wear, corrosion, and their interactions is crucial. This study focuses on understanding the deformation mechanisms of NiTiNOL60, a nickel-rich nickel-titanium alloy, during sliding against Al2O3 in different corrosive environments, including acidic, alkaline, and saline mediums. The pH of the environments is found to play a significant role in the tribocorrosion process, leading to electromechanically induced transformations and various wear patterns. Plastic deformations are observed on the wear track surfaces, particularly in the severe and mild wear regimes. In an alkaline environment, depassivation of the oxide layer triggers oxidational wear, with the depassivation rate dependent on factors like contact pressure, sliding velocity, and passive film properties. The wear volume is highest in saline environments, with contributions from mechanical wear, corrosion, and third-body abrasion. Grain deformations occur in the alkaline environment due to shear forces, while in the acidic medium, corrosion accelerates mild wear involving abrasion and delamination. The findings provide insights into wear mechanisms and localized corrosion, highlighting the influence of H+ and OH− groups (pH values) on corrosive wear and crack propagation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nickel-titanium (Ni-Ti)-based alloys are being increasingly explored for use in a range of industries (Ref 1) and have, thus, garnered considerable scientific attention due to their unique shape memory and mechanical properties. This study concentrates on NiTiNOL60, also referred to as 60NiTi, which is composed of 60 wt.% Ni and 40 wt.% Ti. NiTiNOL60 possesses unique properties such as high specific strength, low density, biocompatibility, superelasticity, machinability, and good corrosion resistance, which make it suitable for a wide range of applications in marine, aerospace, biomedical, and food processing industries (Ref 2, 3). It is an ordered intermetallic material compound consisting of a B2, cubic crystal structure matrix with one or more secondary phases, which includes the metastable NiTi and Ni3Ti2 phases and the equilibrium Ni3Ti phase, also referred to as the globular Ni3Ti precipitates. The binary NiTiNOL60 alloy has been shown to achieve the highest hardness among the binary compounds after heat treatment at 1050 °C (Ref 4). According to Xu, et al. Ref 5, the high hardness is caused by precipitation hardening through the rapid formation of nanoscale Ni4Ti3 precipitates upon cooling from a solution-annealed condition. As shown in Figure 1, the Ni4Ti3 precipitates form inside narrow B2 NiTi channels and cause an Orowan strengthening mechanism (Ref 1, 4). These properties are due to the intrinsic thermoelastic martensite transformation under thermal and mechanical loading conditions at different temperatures (Ref 6). At relatively low temperatures and under external force, the initial parent phase (austenite) transforms into a martensite phase accompanied by macroscopic deformation (Ref 7).
Several researchers have investigated the properties of this alloy to better understand the desired approach to enhancing the durability and efficiency of the systems in which it is employed. While Yan, et al. (Ref 8) studied the microstructure and tribocorrosion performance of NiTiNOL60 alloy in artificial seawater, Kosec, et al. (Ref 9) investigated the effects of different microstructures on tribocorrosion behavior of NiTi alloy in simulated saliva. In their findings, B2 TiNi and stable TiNi3 phases were identified for the as-cast NiTiNOL60 sample, but solution treatment could lead to the precipitation of metastable Ti3Ni4 phase in the matrix without dissolving the TiNi3 phase completely. This confirms the Orowan strengthening mechanism presented in Figure 1 (Ref 10).
For space-bearing applications, DellaCorte (Ref 11) and Pepper, et al. (Ref 12) investigated the superelastic behavior of NiTiNOL60 alloy. According to DellaCorte (Ref 13), 60NiTi is highly elastic, unaffected by rust, combines high hardness, reduced stiffness and superelasticity, and has a higher load-carrying capacity than steel. However, NiTiNOL60 and many other metals under certain mechanical loading and harsh corrosive conditions deteriorate, which warrants the evaluation of their wear performance (Ref 14). Accordingly, special attention has been paid to remedy the tribological contact using thick barrier coatings and other surface treatments, yet metal/alloy failures prevail. NiTiNOL60 alloy is tribochemically benign in the presence of liquid lubricants, compared to conventional alloys with large concentrations of titanium that proved to be chemically aggressive and cause degradation of many lubricants (Ref 11, 15). Khanlari, et al. (Ref 16) investigated the unlubricated sliding wear performance of 60NiTi compared to 440C steel. Their study revealed that 440C steel exhibits more microscopic plasticity than 60NiTi, which hindered the propagation of generated tensile microcracks. Furthermore, Zeng and Dong (Ref 17) studied the surface roughness behaviors of the alloy under oil lubrication, and their findings showed a remarkable lubrication performance with an ultra-low coefficient of friction of about 0.008 maintained under castor oil lubrication (Ref 18, 19).
Pertaining to reports in the literature that mechanical loading and extreme corrosive conditions increase surface deterioration, this study focuses on a thorough investigation of the tribocorrosion performance of NiTiNOL60 at different corrosive mediums. While Kosec, et al. (Ref 9) have categorized the NiTi research conducted so far into the following fields: basic corrosion and electrochemical studies, mechanical and material property studies, and surface treatment studies, the comparative study of the tribocorrosion behavior of NiTiNOL60 in different chemical environments has remained unexplored.
It is known that corrosion is accelerated when combined with mechanical wear (Ref 9). Tribocorrosion is an irreversible material transformation (surface damage) occurring from the simultaneous action of wear and corrosion due to relatively moving mechanical contact and electrochemical reactions (Ref 20,21,22). Wang, et al. (Ref 23) studied the tribocorrosion behavior of titanium alloys in simulated body fluids, and their findings demonstrated that the applied load and concentration of wear particles had important effects on tribocorrosion behavior. Despite the reported high resistance to wear exhibited by NiTi alloy, an investigation by Guadalupe, et al. Ref 21 compared the tribological behavior of NiTi alloy to conventional engineering materials such as steels (Ref 24), Ni-based (Ref 25), and stellite (Ref 26, 27) alloys and reported that the wear resistance of conventional tribomaterials strongly depends on their mechanical properties such as hardness, toughness, and work hardening. In addition, a study by Muñoz and Espallargas (Ref 28) highlighted that mechanical parameters strongly influence the electrochemical material removal rate, and, on the other hand, the mechanical material removal rate depends on the prevailing electrochemical conditions. For electrochemical investigation, it is established that the techniques employed in tribocorrosion rely on the contact geometries of the samples (Ref 29). In the case of a linear reciprocating ball-on-plate configuration, suitable methods include open-circuit potential (OCP), potentiodynamic polarization (PD), potentiostatic polarization (PS), and electrochemical impedance spectroscopy (EIS) (Ref 30). During an electrochemical reaction, the metal undergoes oxidation by the oxidizing agent. Several factors influence the corrosion process, such as the metal's position in the electrochemical series, pH, presence of electrolytes, impurities in the metal, concentration of oxygen, and temperature differences (Ref 31). The material exhibits different electrochemical responses depending on its state: immune state (no corrosion), active state (corrosion resulting in metal loss or dissolution of one of the constituents of the corrosive environment into the metal), and passive state (the metal forms a protective oxide passive film on its surface, reducing the corrosion rate). Metals with low corrosion potentials experience anodic polarization, leading to an increasing anodic dissolution rate. On the other hand, metals with high corrosion potentials undergo cathodic polarization, which slows down the dissolution rate (Ref 32).
Given the varied behaviors of the material under different environments, it is crucial to thoroughly examine and compare the tribocorrosion phenomena and electrochemical interactions of NiTiNOL60 alloy in different corrosive mediums across a wide pH range. By exploring these aspects, this study aims to provide valuable insights into the tribocorrosion behavior of NiTiNOL60, facilitating the development of effective strategies to enhance durability and efficiency in applications where this alloy is employed.
2 Materials and Methods
NiTiNOL60 plates were obtained from the National Aeronautics and Space Administration (NASA), Glenn Research Centre in Cleveland, Ohio, USA. The samples were manufactured through the Hot Isostatic Pressing (HIP) method, and the manufacturing details can be found in (Ref 33). The NiTiNOL60 plates with the dimensions of 10 mm × 10 mm × 20 mm were used for the tribocorrosion tests. The preparation of the plates before tribocorrosion testing involved wet grinding of the samples using a Buehler-EcoMet 30 machine and silicon carbide (SiC) #180, 500, 1200, and 2400-grit sandpapers having corresponding grain sizes of 104, 35, 18, and 8 µm, respectively. Subsequently, the mechanical polishing of the samples was carried out using 6-µm and 1-µm monocrystalline diamond paste (DP-paste M) on a polishing surface of the Struers-Labopol-2 machine for a good surface finish. The polished samples were subjected to ultrasonic cleaning with absolute ethanol, and the surfaces were characterized using an optical microscope (AMScope-Olympus BX51M model) to examine the microstructure of the sample. Hitachi SU-70 scanning electron microscope (SEM) and energy-dispersive spectroscopy (EDS) were used to ascertain the samples’ morphology and elemental composition before subjecting the sample to mechanical loading in a corrosive environment. A working electrode terminal was connected to the dry plate using a non-conductive copper tape. Only the polished surface to be examined was exposed, while the other sides of the plate were coated with a Plasti-Dip paint to ensure their non-interference during the experiments. The Plasti-Dip is a fast-drying multi-purpose rubber coating that adheres firmly to the target surface and protects the underlying metal against corrosive environments. According to Mathew and Wimmer (Ref 34), one of the contacting bodies is kept as an insulator and the conductive body is used as the testing sample for tribocorrosive analyses.
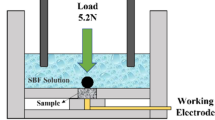
For tribocorrosion investigation, the tests involved the coupling of a linear reciprocating tribometer (DUCOM TR-282 model) and an electrochemical cell workstation (IviumStat potentiostat) of a 3-electrode configuration (Ref 30) shown in Figure 2. The experimental setup and procedures adopted for this research are based on the standard test methods for linear reciprocating ball-on-flat sliding (Ref 35), the electrochemical measurements in linear reciprocating contact (Ref 36, 37), and the synergy between wear and corrosion ASTM G119-09 (Ref 38). The prepared samples were placed firmly in the holder of the linear reciprocating tribometer for the simultaneous sliding and electrochemical interactions (Ref 39). Table 1 shows the test parameters for the tribocorrosion measurements.
Figure 2 shows an experimental setup of a three-electrode configuration connected in series to two computer screens for data registration. WINDUCOM and IviumSoft software tools were used for tribological and electrochemical measurements, respectively. For the testing, three different electrolyte solutions with concentrations of 0.05 M H2SO4, 0.05 M NaOH, and 3.5 wt.% NaCl were employed. The corresponding pH values of the solutions were 1.4, 6.7, and 12.4, respectively. Using the ball-on-plate configuration allowed a clamped counter body, an alumina (Al2O3) ball with Ø 10 mm diameter, to slide against the polished surface of the NiTiNOL60 sample under specific test conditions at room temperature. For each sliding wear test, a fresh surface of the Al2O3 ball is revealed to allow free rubbing and avoid debris adhesion interferences on the ball-worn surfaces. Graphite rod and calomel electrodes were used as the counter electrode (CE) and reference electrode (RE), respectively, while the prepared NiTiNOL60 specimen with an exposed surface area of 2 cm2 acted as the working electrode (WE) and fully immersed in the testing solution. Before the potentiodynamic polarization measurements, the specimen was allowed to stabilize in the open-circuit potential mode for 20 minutes. Afterward, simultaneous reciprocating sliding and polarization measurements were taken at the set electrochemical potential voltage and scan rate of 2 mV/s. During the test, the sliding frequency was constant at 4 Hz under different applied normal loads of 2 N, 5N, and 8N, as shown in Table 1. A stroke length of 10 mm was applied, covering a total sliding distance of 500 m. At the end of each measurement, the system was allowed a re-conditioning time of 20 min. Afterward, the sample was carefully removed and cleaned ultrasonically for surface examination. Each test was repeated three times, and the average values of the results were reported and analyzed.
The wear tracks on the surfaces were examined after the tribocorrosion tests using an optical microscope (AMScope-Olympus BX51M model) to observe the microstructure of the sample. Scanning electron microscope (SEM-Hitachi SU-70 model) was also used to ascertain the morphology on the wear track, and energy dispersion spectroscopy (EDS-Hitachi SU-70 model) was utilized for the elemental compositions analysis of debris adhesions on the wear track. Further, hardness tests were conducted using a Rockwell Hardness tester (HR-150A) and a microhardness tester (Leco LM-800AT). For the macroindentation hardness tests, a combination of major and minor loads (140 + 10) of 150 kg was applied, and the resulting values were recorded. For the microhardness tests, the specimen was clamped, focused, and tested with 1.0 kgf with a dwell time of 10 seconds. Then, the hardness was calculated by measuring the geometrical aspect of the indentation, including the surface area and depth of the diamond shape. A Taylor Hobson stylus profilometer (Talysurf 50 model) was used for roughness measurements, as well as measuring the wear volumes at different applied loads. Five different areas of the wear tracks were examined for the wear volume measurements. The measurements are obtained as the stylus tip engages the measuring surface by moving vertically along the wear track and tracing the desired path to determine the topography of the surface. The resulting profile is an established two-dimensional output of a surface height and distance along the measured line. The output information is obtained as a graphical representation showing the surface (wear track) roughness, waviness, grooves, and roughness profile.
To calculate the area of the wear scar, ImageJ software was used to estimate the average width and depth of the wear profiles. The estimated parameters were then used to calculate the area of the wear track, while the volume was obtained by multiplying the area with the stroke length (i.e., the wear track length) of 10 mm. With the obtained wear volume, we calculated the specific wear rate using Eq. 1.
The wear rate quantifies the amount of material worn under specific operating conditions per unit time. The coefficient of friction (CoF) can be calculated using Eq 2:
where μ is the coefficient of friction, Ft is the tangential force, and Fn is the normal load of the desired moving body.
Hutchings 40 expressed that the wear volume of the material during the sliding wear process can be represented as shown in Eq 3:
where Vmech = wear volume due to sliding, L = total sliding distance, Fn = applied normal load, H = hardness of the material, and K = wear coefficient.
Yan, et al. 8 showed that the wear volume recorded from tribocorrosion tests is due to the combined actions of sliding wear and the electrochemical interactions. Thus, to calculate the volume loss due to the combined actions of corrosion and mechanical wear, i.e., the total wear volume (Vt) as the sum of the material loss due to sliding wear (Vmech) and the loss of material due to corrosion or electrochemical oxidation (Vchem), the synergistic wear can be quantified using Eq 4.
Following the synergistic approach and the induced transformations caused by reciprocating sliding, it is necessary to investigate the subsurface of the specimen. After the sliding wear tests in a corrosive medium, the specimen was cut through the wear track using wire cutting to obtain a cross-sectional part of the specimen which was mounted using phenolic hot mounting resin. The resulting surface was mechanically ground and wet polished in preparation for etching. The etching was carried out for about 30 seconds using Kroll’s reagent: 87.7% H2O, 14.1% HNO3, and 3.2% HF and characterized to reveal the subsurface deformations. The schematic representation of the mounted sample as well as the sample geometries before and after tribocorrosion is shown in Figure 3.
3 Results and Discussion
Since the tribological contacts involve the simultaneous wear and corrosion processes, leading to irreversible material transformations (Ref 41, 42), various factors influence the rate of wear and corrosion, and their synergistic interactions need to be analyzed to understand the contribution of each process to material removal (Ref 43). Therefore, it is essential to critically examine the wear track using techniques such as optical microscopy, scanning electron microscopy (SEM), and energy-dispersive x-ray spectroscopy (EDS) to assess surface topography and subsurface microstructures. Thus, the polished samples with mirror surface finishes were examined using scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) for a full scan at 20 KV penetrating voltage. This ensures that the x-rays comprehensively analyze the sample’s surface and subsurface. Figure 4 shows the NiTiNOL60 alloy’s micrograph and the elemental quantitative compositions obtained from SEM/EDS examination. From the results, it is evident that the alloy investigated has a quantitative elemental distribution of 60 wt.% Ni and 40 wt.% Ti, which is consistent with the observations in the literature (Ref 4, 44). Further examination with the optical microscope presents the micrographs in Figure 5. The images reveal that the sample is an ordered intermetallic compound of a cubic crystal matrix structure. This confirms the observations in the literature by previous researchers (Ref 45, 46). The micrographs show clear grain boundaries with cluster particles of the microstructural grains in the consolidated and unconsolidated regions. The regions confirm the composition of the sample, indicating a dominance of the B2 NiTi matrix and Ni4Ti3 phases, which could be referred to as cubic and rhombohedral crystal structures (Ref 45, 47).
The hardness of the NiTiNOL60 sample was measured before and after tribocorrosion tests in different electrolytes. From the measurements, an average value of the Rockwell hardness of 62.5 HRC was recorded for the plain/unused sample, which agrees with existing literature information (Ref 24), whereas the microhardness measurements of the same sample recorded an average value of Hv 537.77/1.0kgf/10 s. Microhardness tests were carried out along the wear tracks of the samples subjected to tribocorrosion tests, while the Rockwell hardness tester was used to measure the macroindentation hardness as specified in Section 2. For each specimen, the average hardness was obtained by performing five tests on different parts of the surface and the resulting values are shown in Table 2. The results show that the overall hardness of the sample increased after tribocorrosion and this is an indication that the continuous sliding actions led to damages on both the surface and crystal structures. Strain hardening of solid crystals involves a modification of the structure due to plastic deformation which creates dislocation and increases strength at the induced surface. Our analysis confirmed that the sample immersed in the saline solution gained the highest hardness with a value of 67 HRC which showed that salt has the chemical effect of decomposing the elements. In contrast, the sample in the acidic environment has the least value, 63.3 HRC, which is slightly higher than the original value of the plain sample. The increased hardness recorded for the specimens exposed to the three corrosive environments could be attributed to different factors, including work hardening during the reciprocating sliding, oxide layer formation, and debris adhesion along the wear tracks. The strain hardening resulting from compression during the reciprocating sliding significantly altered the microstructure of the specimen. This is evident in SEM micrographs revealing the surface and subsurface damages which occurred due to plastic deformation. This aligns with literature information which presents that plastic deformation increases the dislocation density in the crystal structures 48, 49. In another study, Bounezour, et al. 49 highlighted that work hardening promotes surface hardness (Hv) and high tendencies of increased brittleness as cracks on the SEM images show.
Figure 6 presents the evolution of the friction coefficient during sliding. The initial sliding stage is characterized by frictional interactions that result in localized heating and oxidation of asperities, leading to oxidational wear (Ref 50). It is well-established that surface roughness plays a crucial role in frictional behavior, manifesting in various forms during the sliding contact. The plotted data clearly indicate that the coefficient of friction (CoF) increases with higher applied loads across the three different mediums investigated. Consequently, the increased load induces more significant wear and activates distinct wear mechanisms along the wear track, as will be confirmed by the subsequent SEM images. Notably, the CoF values at higher loads of 5N and 8N demonstrate that elevated contact pressures intensify wear surface roughness and broaden the wear track compared to the lower applied load of 2N (Figure 14d). The CoF plot for the 2N load exhibits a running-in period of approximately 500 s for the sample tested in the saline and alkaline environments. In contrast, the sample tested in the acidic environments recorded a longer running-in time, following consistent wear of the material’s surface. This suggests that the applied load of 2N was insufficient to penetrate and remove the passive layer through wear. The plots for the 2N load show less noise during sliding, confirming little or no adhesion and less interference from the wear debris. However, as the counter material continuously slides against the NiTiNOL60 surface, the passive oxide layer gradually wears off, allowing penetration into the sample surface. After the running-in period, the CoF increases and reaches a relatively stable friction coefficient, primarily attributed to the general oxidation of the sliding surfaces. Conversely, at higher loads, the running-in period is negligible, indicating that the contact pressure from the applied load is adequate to break down the passive layer through the reciprocating sliding action of the alumina ball (Ref 17). This process induces surface roughness and triggers various wear mechanisms until the system reaches a relatively steady state characterized by a high CoF. Unlike in the other environments, the CoF trend recorded for higher loads in the H2SO4 environment shows a continual decline. This suggests less adhesion and relatively little or no impact from the third-body abrasion as sliding progressed.
To better understand the tribocorrosion behavior of NiTiNOL60 under different applied loads and electrochemical conditions, a detailed examination of the wear track was performed. The SEM analysis revealed the occurrence of various wear mechanisms on the NiTiNOL60 surface, including abrasion, adhesion, delamination, ploughing, pitting (nucleation and metastable), crevices, and cracks perpendicular to the sliding direction, as depicted in Figure 7. During sliding, adhesive wear was observed due to micro-joint formation between the Al2O3 ball and the polished NiTiNOL60 surface (Ref 40). Furthermore, surface fatigue was evident at the 8N load, particularly in the saline environment, which could be attributed to cyclic loading induced by reciprocating sliding. Abrasive wear occurred as ploughing or cracking at the interface between the counter material and the NiTiNOL60 surface, with severity varying based on the applied load and corresponding contact pressure. The wear behavior exhibited under different regimes suggests that corrosion-induced wear is typically accelerated by oxidation in a corrosive environment, with the extent influenced by the pH of the system. While pitting corrosion may seem less significant, its depth and propagation rate can lead to rapid metal dissolution if the active state persists for an extended duration (Ref 51). The synergistic effects of tribocorrosion revealed that material deformation occurs in various forms depending on the environmental conditions. In alkaline and saline environments with higher pH ranges, oxidational wear leads to mild to severe surface damage, potentially resulting in plastic deformation. In contrast, acidic environments promote abrasion, delamination, and adhesion, resulting in milder wear. The SEM images, particularly under the 8N load, demonstrate that the applied load or contact pressure generates a larger wear track and increased surface roughness, consequently influencing deformation and friction by enhancing asperity deformation. In contrast, lower surface roughness favors adhesive friction. Comparatively, non-unidirectional abrasive wear grooves resulting from third-body wear are more prevalent in the NaOH environment, while the NaCl environment exhibits the highest wear volume among the three investigated environments due to the combined effects of mechanical and chemical wear. This finding confirms the significant role of solution anions, including halide and non-halide ions, in the growth and breakdown of the passive film in aqueous solutions (Ref 51). The NiTiNOL60 samples exposed to the H2SO4 environment exhibited the least wear volume from mechanical wear. The wear debris from the alumina ball was observed to be relatively soluble in the electrolyte solution. The elemental quantification using energy-dispersive x-ray spectroscopy (EDS) further supported the dissolution of alumina particles, with fewer traces of Al debris detected along the wear track in the acidic medium compared to the debris accumulation and adhesion observed in the NaOH and NaCl environments. Additionally, as shown in Figure 6, the CoF curves for H2SO4 at 5N and 8N loads gradually decreased as the sliding progressed. This drop could be attributed to the dissolution of alumina wear debris and general oxidation of the interfacial surfaces.
As stated above, further sliding produces more fragments, which promotes adhesion (Ref 52); on that note, our EDS examination of the wear tracks revealed the elemental compositions of the examined surfaces. The results presented in Figure 8 confirmed that the wear track surfaces were oxidized during tribocorrosion. The spectrum highlights the level of concentrations of each identified element. The higher the peak in a spectrum, the more concentrated the element in the specimen. The results show a high concentration of the NiTiNOL60 base material (Ni and Ti) followed by aluminum and oxygen concentrations resulting from the adhesion of the counter material debris and surface oxidation, while an area scan shows that the sample exposed to NaOH recorded a distributed oxidized composition and the highest amount of Al debris, which contributed to the third-body wear abrasion (Ref 53), as seen in the SEM images. The sample exposed to the acidic medium recorded the least distribution of Al2O3 debris when compared with the samples exposed to other corrosive environments. This supports the unidirectional wear groves shown in the SEM images in Figure 7, with less impact from the third-body effect (Ref 53), which eventually resulted in the lowest wear volumes recorded at different test conditions except for the 2N load. In support of the quantitative elemental distribution, the EDS spectrum shows that Al has the lowest peak, thereby confirming a lower concentration of alumina debris in the acidic environment. According to the data for elemental distribution, the sample tested in a NaOH environment had the maximum oxygen concentration at 8N load with 23.93 %, followed by the saline environment at 13.66 % and the acidic environment at 9.02 %. This finding for the NaOH medium could also be supported by the high oxidation level in Figure 7, showing different corrosion attacks for the SEM image of 8N NaOH. In the saline environment, the 13.66 % oxygen concentration is believed to significantly support the halide ions (Cl−) in contributing to the overall corrosion rate for the NaCl environment.
Following the wear mechanisms at the sliding interfaces and the high level of Al debris adhesion observed on the sample surfaces in Figure 8, we further examined the surfaces of the counter body (alumina balls) sliding against the NiTiNOL60 specimens after tribocorrosion. Figure 9 presents the EDS analysis of the examined Al2O3 ball surfaces used for reciprocating sliding in the three different environments. The results confirm an adhesion mechanism on the counter body surface having NiTiNOL60 debris distributed at different compositions. From the results, point 1 gives the quantitative elemental distribution of an area/overall scan showing the base material of the ball (Al and O) as well as the composition of the specimen (Ni and Ti). Analyses of the results show that the sample exposed to an acidic medium recorded the least percentage of Ni-Ti adhesion; however, the sample immersed in the saline environment has the highest amount of Ni-Ti debris adhesion onto the ball surface which conforms to the highest wear volume and CoF recorded for the different applied loads in NaCl environment. It also supports the adhesion mechanism highlighted in Figure 7 for the NaCl environment. Additionally, the similar concentrations of oxygen presented in the quantitative results for the examined surfaces in the three corrosive environments also confirm the oxidative wear mechanism at both contact surfaces.
Considering the electrochemical reactions occurring on the material's surface during tribocorrosion, the activities involve anodic oxidation (removal of electrons from metal atoms) and cathodic reduction (consumption of electrons by the oxidizing agent). These reactions are elucidated by analyzing the cathodic and anodic regions of the Tafel plots in Figure 10 and used to estimate the potentiodynamic polarization parameters presented in Table 3. The Tafel plot illustrates the occurrence of corrosion in both the anodic and cathodic regions, revealing the sample’s susceptibility to pitting within the saline environment. In contrast, corrosion was primarily observed in the cathodic region in the acidic and alkaline environments. It reveals that localized corrosion initiated and progressed in both anodic and cathodic regions after reaching equilibrium for lower loads (2N), where the contact pressure is insufficient to wear away the passive layer. This indicates that the sample surfaces experience anodic polarization at lower loads with an increased dissolution rate in that region due to their low corrosion potential. Conversely, for higher applied loads (8N), the material surfaces undergo cathodic polarization at a higher corrosion potential, which slows down the dissolution rate. Accordingly, this leads to an increased corrosion rate, higher wear volume due to corrosion and severe pitting corrosion in saline medium, as presented in Figure 10. The summarized potentiodynamic polarization parameters estimated from the Tafel plots are provided in Table 3, offering an overview of the electrochemical behavior during the tribocorrosion testing. From the tabulated results, it is evident that an increase in the applied load decreases the polarization resistance (Rp). This increases the corrosion current (Icorr) and shifts the corrosion potential (Ecorr) toward the passive direction (i.e., toward zero or to more positive values). The parameters deduced for the sample investigated in the saline environment recorded the highest corrosion rate, the highest corrosion current density, and the lowest polarization resistance when compared with the samples tested in the acid and alkaline environments. Equally, the parameters obtained for the sample tested in the acidic medium presented the highest values of polarization resistance; this invariably results in the lowest corrosion rates for the different applied loads. Whereas the alkaline environment depicted the least values for the corrosion current density and corrosion potential at different loads, the polarization resistance was higher compared to the values obtained in the 3.5 wt.% NaCl environment. Overall, the results agree with the previous reports stating that the lcorr value denotes a lower corrosion rate (Ref 54).
Notably, halide ions, such as Cl−, contribute to severe localized corrosion, including pitting and cracks observed in the saline environment. The different stages of localized corrosion reported can be seen in the SEM images shown in Figure 11 and 12, while Figure 11 shows pit nucleation and its propagation, as well as the advancement of corrosion crevices. Figure 12 (a) and (b) shows the pit formation and microcrack propagation in a sliding direction in the NaOH environment before and after etching. Etching of the sample clearly highlights a stable pitting, where the pit and crack propagated beyond the surface of the sample down to about 120 µm, unlike in the other environments where the localized corrosion (pitting) only maintained the nucleation and metastable stages. The continuous reciprocating sliding along the wear track and in the localized corrosion regime induced fatigue wear and promoted stress corrosion. The stress corrosion which was aggravated from the combined influence of compressive stresses, increased the dislocation density in the crystal structures. Hence, the increased pitting and crevices as well as the elongated at both minimum and maximum contact pressures. Conversely, the severe corrosion leading to stress corrosion results from the relative motion of a corrosive fluid on the material surface. Pitting, the conventional corrosion form, exists in different stages even though pitting is self-initiating and self-propagating, making it difficult to determine the borders for every stage, including the nucleation, metastable pit formation, and stable pitting (Ref 51). Corrosion is typically initiated at defects in the protective oxide film or at the break of the passive film and progresses by forming a dense tubercle of hard rust or scale, with pits present in the underlying metal (Ref 51). According to Ghali Ref 51, the propagation emanating from the pits could result in fatigue corrosion and stress-corrosion cracking. For the environments investigated, there exist similarities in the corrosion mechanism since the localized corrosion failures could be attributed to mechanically assisted corrosion despite the variations in the initiation of pitting and crevice (Ref 55). According to Ghali Ref 51, pitting corrosion is commonly observed in the presence of chlorides and halides, and this is evident in the results obtained where pitting corrosion occurred more in the NaCl environment compared to the other environments. As observed in this investigation, continuous sliding actions at higher loads easily destroy the protective properties of the passive film, thereby exposing more layers of the material surface and resulting in a high corrosion rate before the surface repassivates (Ref 31, 56).
Continuous sliding at an 8N load in the NaOH environment resulted in grain elongation and stress-corrosion cracking due to the wear-accelerated corrosion resulting from the tribocorrosion synergy. Accordingly, Figure 13 presents the cross section of the sample, revealing surface damages and distortion in the microstructural arrangement. From the images, it could be deduced that sliding action caused mild to severe surface damages, and the third-body effect from the resulting debris led to a non-uniform direction in the wear track abrasion grooves, thereby causing wear roughening and wear anisotropy (Ref 53, 57). The shear force possibly caused the grain structures to be flattened and elongated longitudinally in the sliding direction, with irregularly shaped eutectic and dendritic growth (Ref 2). This impact could further lead to plastic deformation because of further compression and shear due to sliding and altering the martensite phase comparable to the eutectic and dendritic network structures. Further sliding results in the formation of additional fragments, which then adhere to the original fragment and finally delaminate to produce a much larger conglomerate wear particle. Therefore, the wear mechanisms and/or patterns are significantly influenced by the equilibrium between the depassivation and repassivation rates. This is reinforced by the findings of Ferreira, et al. Ref 58, who reported that the depassivation rate depends on the contact pressure, the sliding velocity, the counter body, and mechanical properties of the passive film, such as scratch resistance (Ref 24). Additionally, we have deduced that under tribocorrosion conditions, the synergistic interactions of wear accelerated corrosive and material loss due to sliding contact commonly affect the depassivation mechanisms during the contact surface damage.
Figure 14 (a, b, c, and d) presents the summarized relationships between the applied normal loads and corrosion rate (Figure 14a), wear volume (Figure 14b), specific wear rate (Figure 14c), and the wear track profile (Figure 14d). Figure 14d shows the profiles for samples tested in the acidic medium where the minimum wear was recorded. From the profiles, wear volumes at different loads were estimated using a stylus profilometer and ImageJ software as outlined in section 2, and the specific wear rate was calculated using Eq 1. It demonstrates that as the applied load increases, the wear track indentation increases, leading to a higher wear volume. From the results, it is clear that mechanical and chemically induced wear was highest for the sample investigated in the NaCl environment when compared to the samples tested in the acid and alkali environments. Thus, an indication of the significant role played by halides in passive film growth and breakdown. This demonstrates that metals or ions are reduced cathodically during the electrochemical breakdown of various metal oxides (Ref 51). The results highlighted the prevalence of corrosion in all the investigated environments, indicating that fretting corrosion involves both chemical and mechanical factors, with observed damage arising from a combination of these factors (Ref 28, 59, 60). The mechanical abrasion of the passive film often leads to wear-accelerated corrosion due to the high chemical reactivity of the bare metal surface exposed to the corrosive environment (Ref 61). Additionally, passive films significantly influence the surface mechanical response of metals and the behavior of the third body in the tribological system (Ref 31, 53, 62). The progressive increase in the pattern of wear rate shown in Figure 14 confirms wear acceleration due to the simultaneous electrochemical–mechanical interactions during tribocorrosion. Accordingly, the findings from previous researchers stated that under tribocorrosion conditions, two main mechanisms contribute to material removal from the surface: wear-accelerated corrosion and mechanical removal through sliding contact (Ref 28, 52, 63). According to Yan, et al. Ref 8, the mechanistic approach distinguishes two primary contributions: anodic dissolution (wear-accelerated corrosion) and mechanical removal of metal particles (mechanical wear) (Ref 8, 60). Overall, Figure 14 illustrates that as the applied load increased, the wear rate significantly increased. The sample tested in the H2SO4 environment maintained the least wear volume, except for the test condition of 2N load, where the sample yielded more than the sample tested in a NaOH environment. This could be attributed to the prolonged running-in period shown in Figure 6, which impacted the material loss through mechanical removal due to inadequate contact pressure from the applied load. Also, the sample tested in the NaOH environment markedly showed the lowest corrosion rate following the running-in time, which delayed the breakdown of the oxide film and reduced the exposure of the sample subsurface.
Following the wear pattern at a lower load due to the less contact pressure, a low wear volume was recorded, and this basically occurred as a result of corrosive wear with little contribution of mechanical wear due to sliding (Ref 64). As the load increases, the contact pressure and wear volume increase. The trend in Figure 14 shows that the highest wear volume was recorded in the NaCl environment, followed by the NaOH and then the H2SO4 environment (H2SO4 < NaOH < NaCl). The drop in the wear rate observed for the H2SO4 environment could be attributed to the solubility of the alumina debris, unlike in the other environments where the particles/debris contributed to the third-body wear abrasion.
4 Conclusions
This study focused on investigating and analyzing the triboelectrochemical interactions of NiTiNOL60 alloy in various corrosive environments under different test conditions. The experimental results clearly demonstrated the synergistic effect of wear and corrosion, leading to an accelerated degradation of the material surface. The investigation revealed that the material deterioration was attributed to the combined influence of wear-accelerated corrosion and mechanical wear resulting from the reciprocating sliding action. While the synergistic approach revealed that mechanical wear is the predominant factor on material loss, it was observed that corrosive wear was more pronounced at higher loads, particularly in the saline environment. Considering the influences of anodic dissolution (wear-accelerated corrosion) and mechanical removal of metal particles (mechanical wear), the mechanistic approach to wear was evident in all three investigated environments, with a greater prevalence in the hydroxyl medium (NaOH) which had the highest pH value. Moreover, severe and mild wear mechanisms were observed more in the NaCl and NaOH environments unlike in the least pH medium (H2SO4), indicating the impact of corrosion on the wear behavior. The mechanical abrasion of the passive film led to wear-accelerated corrosion due to the high chemical reactivity in corrosive mediums. The analysis highlighted the significant role of hydrogen ion (H+) and hydroxyl ion (OH−) concentrations in the electrolytes, particularly in the acidic environment, where the alumina debris dissolved in the medium during tribocorrosion. In conclusion, this investigation provided insights into the triboelectrochemical behavior of NiTiNOL60 alloy. The findings emphasized the importance of considering both wear-accelerated corrosion and mechanical wear when studying material degradation in different corrosive environments. The knowledge gained from this study can contribute to the development of improved materials and strategies for mitigating tribocorrosion-related issues in practical applications.
References
S.H. Mills, R.D. Noebe, C. Dellacorte, B. Amin-Ahmadi, and A.P. Stebner, Development of Nickel-Rich Nickel–Titanium–Hafnium Alloys for Tribological Applications, Shape Memory Superelasticity, 2020, 6(3), p 311–322. https://doi.org/10.1007/s40830-020-00296-w
Z. Du, Z. Hu, Y. Feng, and F. Mo, The Effect of Powder Composition on the Microstructure and Corrosion Resistance of Laser Cladding 60NiTi Alloy Coatings on SS 316L, Metals, 2021, 11(7), p 1104. https://doi.org/10.3390/met11071104
A. Okoani, A. Nand, and M. Ramezani, Tribocorrosion Behaviour of NiTiNOL60 Alloy in an Alkaline Environment, Res. Eng., 2023, 19, p 101305. https://doi.org/10.1016/j.rineng.2023.101305
C. Miller, C. DellaCorte, and M. Zou, Nanomechanical Properties of Hardened 60NiTi, Mater. Sci. Eng. A, 2021, 800, p 14028. https://doi.org/10.1016/j.msea.2020.140284
G.X. Xu, L.J. Zheng, F.X. Zhang and H. Zhang, Influence of Solution Heat Treatment on the Microstructural Evolution and Mechanical Behavior of 60NiTi, J. Alloys Compd., 2019, 775, p 698–706. https://doi.org/10.1016/j.jallcom.2018.10.015
S.H. Mills, C. Dellacorte, R.D. Noebe, M.J. Mills, A.P. Stebner, and B. Amin-Ahmadi, Heat treatment—microstructure—hardness relationships of new nickel-rich nickel-titanium-hafnium alloys developed for tribological applications, Materialia, 2021, 16, p 101064. https://doi.org/10.1016/j.mtla.2021.101064
S. Bao, L. Zhang, H. Peng, Q. Fan, and Y. Wen, Effects of Heat Treatment on Martensitic Transformation and Wear Resistance of As-Cast 60NiTi Alloy, Mater. Res. Express, 2019, 6, p 086573. https://doi.org/10.1088/2053-1591/ab1b7a
C. Yan, Q. Zeng, Y. Xu, and W. He, Microstructure, Phase and Tribocorrosion Behavior of 60NiTi Alloy, Appl. Surf. Sci., 2019, 498, p 143838. https://doi.org/10.1016/j.apsusc.2019.143838
T. Kosec, P. Močnik, and A. Legat, The tribocorrosion Behaviour of NiTi Alloy, Appl. Surf. Sci., 2014, 288, p 727–735. https://doi.org/10.1016/j.apsusc.2013.10.116
S.K. Patel and A. Behera, Evolution of Phases and Their Influence on Shape Memory Effect by Varying Sintering Parameters of NiTi Alloys, Met. Mater. Int., 2022, 28(11), p 2691–2705. https://doi.org/10.1007/s12540-021-01166-5
DellaCorte C., Nickel-Titanium alloys: Corrosion “proof” alloys for space bearing, components and mechanism applications. Presented at the 40th Aerospace Mechanisms Symposium, Cocoa Beach, FL., 12-14 May 2010, Paper presentation, No. E-17269, NASA/TM-2010-216334. [Online]. Available: https://ntrs.nasa.gov/citations/20100021919.
S. V. Pepper, C. DellaCorte, R. D. Noebe, D. R. Hull, and G. Glennon, "Nitinol 60 as a material for spacecraft triboelements. Presented at the 13th European Space Mechanisms and Tribology Symposium - ESMATS 13 Conference, Vienna, Austria, September 22-27, 2009, Conference Paper. [Online]. Available: https://ntrs.nasa.gov/api/citations/20090034488/downloads/20090034488.pdf.
C. DellaCorte, "NiTi alloys for tribological applications: The effects of serendipity on research and development. Presented at the Annual Postdoctoral Research and Career Symposium, October 6th. NASA/ GRC-E-DAA-TN34568, Argonne IL., 2016. [Online]. Available: https://ntrs.nasa.gov/api/citations/20170003993/downloads/20170003993.pdf.
W. Xu et al., Synergistic Interactions Between Wear and Corrosion of Ti-16Mo Orthopedic Alloy, J. Market. Res., 2020, 9(5), p 9996–10003. https://doi.org/10.1016/j.jmrt.2020.06.095
C. Dellacorte, S. V. Pepper, R. Noebe, D. R. Hull, and G. Glennon, "Intermetallic Nickel-Titanium alloys for oil-lubricated bearing applications.," NASA Technical Report. NASA/TM-2009-215646, pp. 1-26, 2009. [Online]. Available: https://ntrs.nasa.gov/api/citations/20090019112/downloads/20090019112.pdf.
K. Khanlari, M. Ramezani, P. Kelly, P. Cao, and T. Neitzert, An investigation on Reasons Causing Inferiority in Unlubricated Sliding Wear Performance of 60NiTi as Compared to 440c Steel, Tribol. Trans., 2019, 62(1), p 96–109. https://doi.org/10.1080/10402004.2018.1516326
Q. Zeng and G. Dong, Influence of Load and Sliding Speed on Super-Low Friction of NiTiNOL 60 Alloy Under Castor Oil Lubrication, Tribol. Lett., 2013, 52(1), p 47–55. https://doi.org/10.1007/s11249-013-0191-1
Q. Zeng and G. Dong, Superlubricity behaviors of Nitinol 60 Alloy Under oil Lubrication, Trans. Nonferrous Met. Soc. China, 2014, 24(2), p 354–359. https://doi.org/10.1016/s1003-6326(14)63068-5
Q. Zeng, G. Dong, and J.M. Martin, Green Superlubricity of Nitinol 60 Alloy Against Steel in Presence of Castor Oil, Sci. Rep., 2016, 6(1), p 29992. https://doi.org/10.1038/srep29992
S. Mischler and A.I. Munoz, Tribocorrosion, Encyclopedia of Interfacial Chemistry. K. Wandelt Ed., Elsevier, Oxford, 2018, p 504–514
M.S. Guadalupe, S. Mischler, M. Cantoni, W.J. Chitty, C. Falcand, and D. Hertz, Mechanical and Chemical Mechanisms in the Tribocorrosion of a Stellite Type Alloy, Wear, 2013, 308(1–2), p 213–221. https://doi.org/10.1016/j.wear.2013.04.007
A. Siddaiah, A. Kasar, R. Ramachandran, and P.L. Menezes, Introduction to Tribocorrosion, Tribocorrosion: Fundamentals, Methods, and Materials, Ch. 1. A. Siddaiah, R. Ramachanran, P.L. Menezes Ed., Academic Press, Cambridge, 2021, p 1–16. https://doi.org/10.1016/B978-0-12-818916-0.00002-X
H. Wang et al., Corrosion Behavior of NiTi Alloy Subjected to Femtosecond Laser Shock Peening Without Protective Coating in air Environment, Appl. Surf. Sci., 2020, 501, p 144338. https://doi.org/10.1016/j.apsusc.2019.144338
K. Khanlari, M. Ramezani, P. Kelly, P. Cao and T. Neitzert, Reciprocating Sliding Wear Behavior of 60NiTi as Compared to 440C Steel Under Lubricated and Unlubricated Conditions, Tribol. Trans., 2018, 61(6), p 991–1002. https://doi.org/10.1080/10402004.2018.1460434
M. Perrut, P. Caron, M. Thomas, and A. Couret, High Temperature Materials for Aerospace Applications: Ni-Based Superalloys and γ-TiAl Alloys, Comptes Rendus Phys., 2018, 19(8), p 657–671. https://doi.org/10.1016/j.crhy.2018.10.002
M.J. Carrington et al., Microstructural Characterisation of Subsurface Deformation and the Degradation of Stellite 6 Induced by Self-Mated Sliding Contact in a Simulated PWR Environment, Tribol. Int., 2021, 158, p 106899. https://doi.org/10.1016/j.triboint.2021.106899
V.L. Ratia et al., Comparison of the Sliding Wear Behaviour of Self-Mated HIPed Stellite 3 and Stellite 6 in a Simulated PWR Water Environment, Wear, 2019, 426–427, p 1222–1232. https://doi.org/10.1016/j.wear.2019.01.116
A.I. Muñoz and N. Espallargas, 5 - Tribocorrosion mechanisms, Sliding Contacts, in Tribocorrosion of Passive Metals and Coatings), Ch. 5. D. Landolt, S. Mischler Ed., Woodhead Publishing, Cambridge, 2011, p 118–152. https://doi.org/10.1533/9780857093738.1.118
M. Azzi and J.E. Klemberg-Sapieha, "8 - Tribocorrosion test protocols for sliding Contacts, Tribocorrosion of Passive Metals and Coatings, Ch. 8. D. Landolt, S. Mischler Ed., Woodhead Publishing, Cambridge, 2011, p 222–238. https://doi.org/10.1533/9780857093738.2.222
D. Landolt, S. Mischler, and M. Stemp, Electrochemical Methods in Tribocorrosion: A Critical Appraisal, Electrochim. Acta, 2001, 46(24–25), p 3913–3929. https://doi.org/10.1016/S0013-4686(01)00679-X
A. López-Ortega, J.L. Arana, and R. Bayón, Tribocorrosion of Passive Materials: A Review on Test Procedures and Standards, Int. J. Corros., 2018, 2018, p 7345346. https://doi.org/10.1155/2018/7345346
J. Yang, Y. Song, K. Dong, and E.-H. Han, Research Progress on the Corrosion Behavior of Titanium Alloys, Corros. Rev., 2023, 41(1), p 5–20. https://doi.org/10.1515/correv-2022-0031
M. K. Stanford, "Hot isostatic pressing of 60-Nitinol," NASA Technical Report. NASA/TM-2015-218884, 2015. [Online]. Available: https://www.techbriefs.com/component/content/article/tb/pub/briefs/manufacturing-prototyping/26497.
M.T. Mathew and M.A. Wimmer, Tribocorrosion in Artificial Joints: In vitro Testing and Clinical Implications, in Bio-Tribocorrosion in Biomaterials and Medical Implants, Woodhead Publishing, Cambridge, 2013, p 341–371. https://doi.org/10.1533/9780857098603.3.341
ASTM G133-22, "Standard test method for linearly reciprocating ball-on-flat sliding wear," American Society for Testing and Materials. Book of standards, vol. 03.02. Developed by subcommittee G02.40, pp. 1-10, 2022, https://doi.org/10.1520/G0133-22.
S.B. Arya and F.J. Joseph, Electrochemical methods tribocorrosion, Tribocorrosion: Fundamentals, Methods, and Materials, Ch. 3. A. Siddaiah, R. Ramachandran, P.L. Menezes Ed., Academic Press, Cambridge, 2021, p 43–77
ASTM G3-14, "Standard practice for conventions applicable to electrochemical measurements in corrosion testing," American Society for Testing and Materials. Book of standards, vol. 03.02. Developed by subcommittee G01.11, pp. 1-9, 2019, https://doi.org/10.1520/G0003-14R19.
ASTM G119-09, "Standard guide for determining synergism between wear and corrosion," American Society for Testing and Materials. Book of standards, vol. 03.02. Developed by subcommittee G02.40, pp. 1-6, 2021, https://doi.org/10.1520/G0119-09R22.
A.I. Munoz, N. Espallargas, and S. Mischler, Experimental techniques for tribocorrosion, Tribocorrosion, Ch. 6. A. Igual Munoz, N. Espallargas, S. Mischler Ed., Springer, Cham, 2020, p 53–64
I. M. Hutchings, Tribology: friction and wear of engineering materials. Boca Raton: Edward Arnold - A division of Hoddr & Stoughton London. CRC Press. ISBN: 0-340-56184-X (in English), 1992.
A. López et al., Tribocorrosion Behaviour of Mooring High Strength Low Alloy Steels in Synthetic Seawater, Wear, 2015, 338–339, p 1–10. https://doi.org/10.1016/j.wear.2015.05.004
S. Alkan and M.S. Gök, Effect of Sliding Wear and Electrochemical Potential on Tribocorrosion Behaviour of AISI 316 Stainless Steel in Seawater, Eng. Sci. Technol. Int. J., 2021, 24(2), p 524–532. https://doi.org/10.1016/j.jestch.2020.07.004
A.I. Munoz, N. Espallargas, and S. Mischler, Tribocorrosion phenomena and concepts, Tribocorrosion, Ch. 4. A. Igualmunoz, N. Espallargas, S. Mischler Ed., Springer, Cham, 2020, p 35–42
M. K. Stanford, "Hardness and microstructure of binary and ternary nitinol compounds," in "NASA Technical Report," National Aeronautics and Space Administration, Glenn Research Center, No. GRC-E-DAA-TN73728, 2016, issue NASA/TM-2016-218946. [Online]. Available: https://ntrs.nasa.gov/citations/20160001689
K. Khanlari, M. Ramezani, P. Kelly, M. Hayat, P. Cao, and T. Neitzert, An Investigation on Microstructural and Mechanical Properties of Porous 60NiTi Parts Solutionized by Different Cost-Effective Methods, Metallogr. Microstruct. Anal., 2018, 7(3), p 334–346. https://doi.org/10.1007/s13632-018-0443-4
O. Benafan, A. Garg, R.D. Noebe, H.D. Skorpenske, K. An, and N. Schell, Deformation Characteristics of the Intermetallic Alloy 60NiTi, Intermetallics, 2017, 82, p 40–52. https://doi.org/10.1016/j.intermet.2016.11.003
K. Khanlari, M. Ramezani, P. Kelly, P. Cao, and T. Neitzert, Mechanical and Microstructural Characteristics of As-Sintered and Solutionized Porous 60NiTi, Intermetallics, 2018, 100, p 32–43. https://doi.org/10.1016/j.intermet.2018.06.001
J. H. Weber, Y. Khalfalla, and K. Y. Benyounis, "Nickel alloys: Thermal treatment and thermomechanical processing," in Reference Module in Materials Science and Materials Engineering, (Elsevier, 2016). https://www.sciencedirect.com/science/article/abs/pii/B9780128035818033828?via%3Dihub
H. Bounezour, L. Laouar, M. Bourbia, and B. Ouzine, Effects of Work Hardening on Mechanical Metal Properties—Experimental Analysis and Simulation by Experiments, Int. J. Adv. Manuf. Technol., 2019, 101(9), p 2475–2485. https://doi.org/10.1007/s00170-018-3071-x
I. Hutchings and P. Shipway, 5—Sliding wear, Tribology, 2nd ed., I. Hutchings, P. Shipway Ed., Butterworth-Heinemann, Oxford, 2017, p 107–164
E. Ghali, Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and Testing (Wiley series in corrosion), Wiley, Hoboken, 2010.
A. Ghanbarzadeh, F.M. Salehi, M. Bryant, and A. Neville, A New Asperity-Scale Mechanistic Model of Tribocorrosive Wear: Synergistic Effects of Mechanical Wear and Corrosion, J. Tribol., 2019, 141(2), p 1–12. https://doi.org/10.1115/1.4041246
D. Landolt, S. Mischler, M. Stemp, and S. Barril, Third Body Effects and Material Fluxes in Tribocorrosion Systems Involving a Sliding Contact, Wear, 2004, 256(5), p 517–524. https://doi.org/10.1016/S0043-1648(03)00561-1
E.B. Caldona, A.C.C. de Leon, J.D. Mangadlao, K.J.A. Lim, B.B. Pajarito, and R.C. Advincula, On the Enhanced Corrosion Resistance of Elastomer-Modified Polybenzoxazine/Graphene Oxide Nanocomposite Coatings, React. Funct. Polym., 2018, 123, p 10–19. https://doi.org/10.1016/j.reactfunctpolym.2017.12.004
A.W. Hansen, L.T. Führ, L.M. Antonini, D.J. Villarinho, C.E.B. Marino, and C.F. Malfatti, The Electrochemical Behavior of the NiTi Alloy in Different Simulated Body Fluids, Mater. Res., 2015, 18(1), p 184–190. https://doi.org/10.1590/1516-1439.305614
J. Chen and F.-Y. Yan, Tribocorrosion Behaviors of Ti–6Al–4V and Monel K500 Alloys Sliding Against 316 Stainless Steel in Artificial Seawater, Trans. Nonferrous Met. Soc. China, 2012, 22(6), p 1356–1365. https://doi.org/10.1016/s1003-6326(11)61326-5
A. Dalmau, C. Richard, and A. Igual-Muñoz, Degradation Mechanisms in Martensitic Stainless Steels: Wear, Corrosion and Tribocorrosion Appraisal, Tribol. Int., 2018, 121, p 167–179. https://doi.org/10.1016/j.triboint.2018.01.036
D.F. Ferreira et al., Synergism Between Mechanical Wear and Corrosion on Tribocorrosion of a Titanium Alloy in a Ringer Solution, J. Market. Res., 2019, 8(2), p 1593–1600. https://doi.org/10.1016/j.jmrt.2018.11.004
Z. Yi et al., Interfacial Friction at Action: Interactions, Regulation, and Applications, Friction, 2023, 11(12), p 2153–2180. https://doi.org/10.1007/s40544-022-0702-x
A.C. Vieira, L.A. Rocha, N. Papageorgiou, and S. Mischler, Mechanical and Electrochemical Deterioration Mechanisms in the Tribocorrosion of Al Alloys in NaCl and in NaNO3 Solutions, Corros. Sci., 2012, 54, p 26–35. https://doi.org/10.1016/j.corsci.2011.08.041
J.P. Celis, P. Ponthiaux, and F. Wenger, Tribo-Corrosion of Materials: Interplay Between Chemical, Electrochemical, and Mechanical Reactivity of Surfaces, Wear, 2006, 261, p 939–946. https://doi.org/10.1016/j.wear.2006.03.027
P. Ponthiaux, F. Wenger, and J.-P. Celis, Tribocorrosion Material Behavior under Combined Conditions of Corrosion and Mechanical Loading, Corros. Res., 2012, 1, p 81–106. https://doi.org/10.5772/35634
A.I. Munoz, N. Espallargas, and S. Mischler, Characterization of worn surfaces, Tribocorrosion, Ch. 7. A. Igual Munoz, N. Espallargas, S. Mischler Ed., Springer, Cham, 2020, p 65–70
R. Neupane and Z. Farhat, Wear Mechanisms of NiTinol under Reciprocating Sliding Contact, Wear, 2014, 315(1–2), p 25–30. https://doi.org/10.1016/j.wear.2014.02.018
Acknowledgment
The authors express their gratitude to Dr. Christopher DellaCorte for generously supplying the NiTiNOL60 samples used in this study for testing and analysis.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okoani, A.O., Nand, A. & Ramezani, M. Comparative Study of the Tribocorrosion Performance of NiTiNOL60 in Acidic, Alkaline, and Saline Environments. J. of Materi Eng and Perform (2024). https://doi.org/10.1007/s11665-024-09646-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-024-09646-6