Abstract
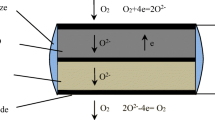
The La0.8Sr0.2(Ga1−x Co x )0.8Mg0.2O3−δ (LSGMC x = 0.05, 0.1, 0.15, 0.2, 0.25) and La0.8Sr0.2(Ga1−x Fe x )0.8Mg0.2O3−δ (LSGMF x = 0.1, 0.2, 0.3) samples were prepared by solid-state reaction. The structure, conductivity, thermal expansion behavior, and chemical compatibility were studied by XRD, dilatometry, and four-terminal method. A limiting current oxygen sensor was prepared with La0.8Sr0.2Ga0.83Mg0.17O2.815 as a solid electrolyte and La0.8Sr0.2(Ga0.75Co0.25)0.8Mg0.2O3−δ as a dense diffusion barrier. The oxygen-sensitive characteristic was measured at different oxygen concentrations. The results show that the phase structure of samples is cubic, except La0.8Sr0.2(Ga0.75Co0.25)0.8Mg0.2O3−δ , which has a hexagonal structure. The change in activation energy for electrical conductivity and the increase in thermal expansion coefficient are confirmed to correlate with an increasing concentration of oxygen vacancies. The limiting current oxygen sensor exhibits a good limiting current platform and the limiting current depends linearly on the oxygen concentration: I L(mA) = 12.8519 + 2.2667 \(x_{{\text{O}_{\text{2}} }}\) (mol%, 0 < \(x_{{{\text{O}}_{ 2} }}\) < 3.31) at 750 °C, I L(mA) = 14.3222 + 3.5180 \(x_{{\text{O}_{\text{2}} }}\) (mol%, 0 < \(x_{{{\text{O}}_{ 2} }}\) < 4.16) at 800 °C, and I L(mA) = 15.2872 + 5.0269\(x_{{\text{O}_{\text{2}} }}\)(mol%, 0 < \(x_{{{\text{O}}_{ 2} }}\) < 4.12) at 850 °C. The sensor has the best sensitivity at 850 °C. As the oxygen concentration increases, the interface resistance of the sensor decreases at 850 °C.









Similar content being viewed by others
References
F. Garzon, I. Raistrick, E. Brosha, R. Houlton, and B.W. Chung, Dense Diffusion Barrier Limiting Current Oxygen Sensors, Sens. Actuators B Chem., 1998, 50, p 125–130
Z.Y. Peng, M.L. Liu, and E. Balko, A New Type of Amperometric Oxygen Sensor Based on a Mixed-Conducting Composite Membrane, Sens. Actuators B Chem., 2001, 72, p 35–40
H. Jiang, J.W. Jian, K. Chen, and Y.Y. Gu, Preparation and Properties of New Dense Diffusion Barrier Limiting Current Oxygen Sensor, J. Chin. Ceram. Soc., 2012, 40, p 1818–1822
B.G. He, T. Liu, J.Z. Guan, and C. Cheng, Preparation and Property of Limiting Current Oxygen Sensor with Sr0.9Y0.1CoO3−δ Dense Diffusion Barrier, J. Chin. Ceram. Soc. Chem., 2012, 42, p 268–274
T. Ishihara, H. Matsuda, and Y. Takita, Doped LaGaO3 Perovskite-Type Oxide as a New Oxide Ionic Conductor, J. Am. Chem. Soc., 1994, 116, p 3801–3803
M. Feng and J.B. Goodenough, A Superior Oxide-Ion Electrolyte, Eur. J. Solid State Inorg. Chem., 1994, 31, p 663–672
T. Ishihara, Perovskite Oxide for Solid Oxide Fuel Cells, Springer, New York, 2009
I. Tadashi and S. Keiichi, Low Temperature Operation of Thin-Film Limiting-Current Type Oxygen Sensor Using Graded-Composition Layer Electrodes, Sens. Actuators B Chem., 2008, 129, p 874–880
J. Mizusaki, Y. Mima, S. Yamauchi, K. Fueki, and H. Tagawa, Nonstoichiometry of the Perovskite-Type Oxides La1−x Sr x CoO3−δ , J. Solid State Chem., 1989, 80, p 102
H. Kishimoto, N. Sakai, T. Horita, K. Yamaji, M.E. Brito et al., Cation Transport Behavior in SOFC Cathode Materials of La0.8Sr0.2CoO3 and La0.8Sr0.2FeO3 with Perovskite Structure, Solid State Ionics, 2007, 178, p 1317–1325
N. Trofimenko and H. Ullmann, Transition Metal Doped Lanthanum Gallates, Solid State Ionics, 1999, 118, p 215–227
F.L. Chen and M.L. Liu, Study of Transition Metal Oxide Doped LaGaO3 as Electrode Materials for LSGM-Based Solid Oxide Fuel Cells, J. Solid State Electrochem., 1998, 3, p 7–14
T. Liu, Y. Li, and J.B. Goodenough, Sr0.7Ho0.3CoO3–δ as a Potential Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cells, J. Power Sources, 2012, 199, p 161–164
L.J. Van der Pauw, A Method of Measuring Specific Resistivity and Hall Effect Of Discs of Arbitrary Shape, Philips Res. Rep., 1958, 13, p 1–9
A. Esquirol, N.P. Brandon, J.A. Kilner, and M. Mogensen, Electrochemical Characterization of La0.6Sr0.4Co0.2Fe0.8O3 Cathodes for Intermediate-Temperature SOFCs, J. Electrochem. Soc., 2004, 151, p A1847–A1855
E. Djurado and M. Labeau, Second Phases in Doped Lanthanum Gallate Perovskites, J. Eur. Ceram. Soc., 1998, 18, p 1397–1404
E.D. Politova, V.V. Aleksandrovskii, G.M. Kaleva, A.V. Mosunov, S.V. Suvorkin, S.V. Zaitsev, J.S. Sung, K.Y. Choo, and T.H. Kim, Mixed conducting Perovskite-Like Ceramics on the Base of Lanthanum Gallate, Solid State Ionics, 2006, 177, p 1779–1783
E.D. Politova, S.Y. Stefanovich, A.K. Avetisov, V.V. Aleksandrovskii, T.Y. Glavatskih, N.V. Golubko, G.M. Kaleva, A.S. Mosunov, and N.U. Venskovskii, Processing, Structure, Microstructure, and Transport Properties of the Oxygen-Conducting Ceramics (La, Sr)(Ga, M)O y (M=Mg, Fe, Ni), J. Solid State Electrochem., 2004, 8, p 655–660
V.V. Kharton, A.P. Viskup, A.A. Yaremchenko, R.T. Baker, G.C. Gharbage, G.C. Mather, F.M. Figueiredo, E.N. Naumovich, and F.M.B. Marques, Ionic Conductivity of La(Sr)Ga(Mg, M)O3−δ (M=Ti, Cr, Fe Co, Ni): Effects Of Transition Metal Dopants, Solid State Ionics, 2000, 132, p 119–130
V.V. Kharton, A.P. Viskup, E.N. Naumovich, and N.M. Lapchuk, Mixed Electronic And Ionic Conductivity of LaCo(M)O3 (M=Ga, Cr, Fe or Ni) I. Oxygen Transport in Perovskites LaCoO3-LaGaO3, Solid State Ionics, 1997, 104, p 67–78
T. Usui, A. Asada, M. Nakazawa, and H. Osanai, Gas Polarographic Oxygen Sensor Using an Oxygen/Zirconia Electrolyte, J. Electrochem. Soc., 1989, 136, p 534–542
K. Saji, H. Kondo, H. Takahashi, T. Takeuchi, and I. Igarashi, Influence of H2O, CO2 and Various Combustible Gases on the Characteristics Of A Limiting Current-Type Oxygen Sensor, J. Appl. Electrochem., 1988, 18, p 757–762
L.S. Darken and R.W. Gurry, Physical Chemistry of Metals, McGraw-Hill, New York, 1953
R. Ramamoorthy, P.K. Dutta, and S.A. Akbar, Oxygen Sensors: Materials, Methods, Designs, and Applications, J. Mater. Sci., 2003, 38, p 4271–4282
E. Ivers-Tiffée, K.H. Härdtl, W. Menesklou, and J. Riegel, Principles of Solid State Oxygen Sensors for Lean Combustion Gas Control, Electrochim. Acta, 2001, 47, p 807–814
J.X. Han, F. Zhou, J.X. Bao, X.J. Wang, and X.W. Song, A High Performance Limiting Current Oxygen Sensor with Ce0.8Sm0.2O1.9 Electrolyte and La0.8Sr0.2Co0.8Fe0.2O3 Diffusion Barrier, Electrochim. Acta, 2013, 108, p 763–768
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51374055, 50904016) and the Fundamental Research Funds for the Central Universities of China (N130502003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, T., Gao, X., He, BG. et al. A Limiting Current Oxygen Sensor Based on LSGM as a Solid Electrolyte and LSGMN (N = Fe, Co) as a Dense Diffusion Barrier. J. of Materi Eng and Perform 25, 2943–2950 (2016). https://doi.org/10.1007/s11665-016-2171-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-016-2171-8