Abstract
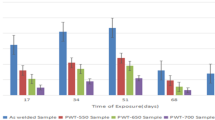
The corrosion resistance of carbon steel weld metal with three different microstructures has been systematically evaluated using electrochemical techniques with the simulated produced water containing CO2 at 90 °C. Microstructures include acicular ferrite, polygonal ferrite, and a small amount of pearlite. With welding heat input increasing, weld metal microstructure becomes more uniform. Electrochemical techniques including potentiodynamic polarization curve, linear polarization resistance, and electrochemical impedance spectroscopy were utilized to characterize the corrosion properties on weld joint, indicating that the best corrosion resistance corresponded to the weld metal with a polygonal ferrite microstructure, whereas the weld metal with the acicular ferrite + polygonal ferrite microstructure showed the worst corrosion resistance. The samples with high welding heat input possessed better corrosion resistance. Results were discussed in terms of crystal plane orientation, grain size, and grain boundary type found in each weld metal by electron backscatter diffraction test.















Similar content being viewed by others
References
Z. Jia, X. Li, C. Du, Z. Liu, and J. Gao, Effect of the Carbon Dioxide Pressure on the Electrochemical Behavior of 3Cr Low Alloyed Steel at High Temperature, Mater. Chem. Phys., 2012, 136, p 973–979
Z. Cui, S. Wu, S. Zhu, and X. Yang, Study on Corrosion Properties of Pipelines in Simulated Produced Water Saturated with Supercritical CO2, Appl. Surf. Sci., 2006, 252, p 2368–2374
Y. Zhang, X. Pang, S. Qu, X. Li, and K. Gao, Discussion of the CO2 Corrosion Mechanism Between Low Partial Pressure and Supercritical Condition, Corros. Sci., 2012, 59, p 186–197
K. George and S. Nešic, Investigation of Carbon Dioxide Corrosion of Mild Steel in the Presence of Acetic Acid-Part 1: Basic Mechanisms, Corrosion., 2007, 63, p 178–186
L. Zhang, X. Li, C. Du, and Y. Cheng, Corrosion and Stress Corrosion Cracking Behavior of X70 Pipeline Steel in a CO2-Containing Solution, J. Mater. Eng. Perform., 2009, 18, p 319–323
M. Kermani and A. Morshed, Carbon Dioxide Corrosion in Oil and Gas Production-A Compendium, Corrosion., 2003, 59, p 659–683
G. Zhang and Y. Cheng, On the Fundamentals of Electrochemical Corrosion of X65 Steel in CO2-Containing Formation Water in the Presence of Acetic Acid in Petroleum Production, Corros. Sci., 2009, 51, p 87–94
F.F. Eliyan, E.-S. Mahdi, and A. Alfantazi, Electrochemical Evaluation of the Corrosion Behaviour of API-X100 Pipeline Steel in Aerated Bicarbonate Solutions, Corros. Sci., 2012, 58, p 181–191
J. Hernandez, A. Muñoz, and J. Genesca, Formation of Iron-Carbonate Scale-Layer and Corrosion Mechanism of API, X70 Pipeline Steel in Carbon Dioxide-Saturated 3% Sodium Chloride, Afinidad, 2012, 69, p 251–258
S. Guo, L. Xu, L. Zhang, W. Chang, and M. Lu, Corrosion of Alloy Steels Containing 2% Chromium in CO2 Environments, Corros. Sci., 2012, 63, p 246–258
C. Avendano-Castro, R. Galvan-Martinez, A. Contreras, M. Salazar, R. Orozco-Cruz, E. Martinez et al., Corrosion Kinetics of Pipeline Carbon Steel Weld Immersed in Aqueous Solution Containing H2S, Corros. Eng. Sci. Technol., 2009, 44, p 149–156
M. Alizadeh and S. Bordbar, The Influence of Microstructure on the Protective Properties of the Corrosion Product Layer Generated on the Welded API, X70 Steel in Chloride Solution, Corros. Sci., 2013, 70, p 170–179
A.R. Shankar, G. Gopalakrishnan, V. Balusamy, and U.K. Mudali, Effect of Heat Input on Microstructural Changes and Corrosion Behavior of Commercially Pure Titanium Welds in Nitric Acid Medium, J. Mater. Eng. Perform., 2009, 18, p 1116–1123
Y. Han, H. Jing, and L. Xu, Welding Heat Input Effect on the Hydrogen Permeation in the X80 Steel Welded Joints, Mater. Chem. Phys., 2012, 132, p 216–222
C. Du, X. Li, P. Liang, Z. Liu, G. Jia, and Y. Cheng, Effects of Microstructure on Corrosion of X70 Pipe Steel in an Alkaline Soil, J. Mater. Eng. Perform., 2009, 18, p 216–220
K. Deen, R. Ahmad, I. Khan, and Z. Farahat, Microstructural Study and Electrochemical Behavior of Low Alloy Steel Weldment, Mater. Des., 2010, 31, p 3051–3055
H.-H. Huang, W.-T. Tsai, and J.-T. Lee, The Influences of Microstructure and Composition on the Electrochemical Behavior of A516 Steel Weldment, Corros. Sci., 1994, 36, p 1027–1038
G. Thewlis, Classification and Quantification of Microstructures in Steels, Mater. Sci. Technol., 2004, 20, p 143–160
J.S. Lee, S.H. Jeong, D.Y. Lim, J.O. Yun, and M.H. Kim, Effects of Welding Heat and Travel Speed on the Impact Property and Microstructure of FC Welds, Met. Mater. Int., 2010, 16, p 827–832
K.-T. Park, S.W. Hwang, J.H. Ji, and C.H. Lee, Inclusions Nucleating Intragranular Polygonal Ferrite and Acicular Ferrite in Low Alloyed Carbon Manganese Steel Welds, Met. Mater. Int., 2011, 17, p 349–356
L. Cui, X. Yang, D. Wang, X. Hou, J. Cao, and W. Xu, Friction Taper Plug Welding for S355 Steel in Underwater Wet Conditions: Welding Performance, Microstructures and Mechanical Properties, Mater. Sci. Eng. A, 2014, 611, p 15–28
O. Grong and D.K. Matlock, Microstructural Development in Mild and Low-Alloy Steel Weld Metals, Int. Met. Rev., 1986, 31, p 27–48
G. Zhang and Y. Cheng, Micro-electrochemical Characterization of Corrosion of Welded X70 Pipeline Steel in Near-Neutral pH Solution, Corros. Sci., 2009, 51, p 1714–1724
H.R. Soleymani and M.E. Ismail, Comparing Corrosion Measurement Methods to Assess the Corrosion Activity of Laboratory OPC and HPC Concrete Specimens, Cem. Concr. Res., 2004, 34, p 2037–2044
H. Al-Mazeedi and R. Cottis, A Practical Evaluation of Electrochemical Noise Parameters as Indicators of Corrosion Type, Electrochim. Acta, 2004, 49, p 2787–2793
M. Pourbaix, Applications of Electrochemistry in Corrosion Science and in Practice, Corros. Sci., 1974, 14, p 25–82
F. Farelas, M. Galicia, B. Brown, S. Nesic, and H. Castaneda, Evolution of Dissolution Processes at the Interface of Carbon Steel Corroding in a CO2 Environment Studied by EIS, Corros. Sci., 2010, 52, p 509–517
S. Nešic and K.-L. Lee, A Mechanistic Model for Carbon Dioxide Corrosion of Mild Steel in the Presence of Protective Iron Carbonate Films-Part 3: Film Growth Model, Corrosion, 2003, 59, p 616–628
J. Zhang, Z.L. Wang, Z.M. Wang, and X. Han, Chemical Analysis of the Initial Corrosion Layer on Pipeline Steels in Simulated CO2-Enhanced Oil Recovery Brines, Corros. Sci., 2012, 65, p 397–404
M.S. Akram and V. Lomadze, On Some Basics of Linear Systems Theory, Syst. Control Lett., 2009, 58, p 83–90
M. Urquidi-Macdonald, S. Real, and D.D. Macdonald, Application of Kramers-Kronig Transforms in the Analysis of Electrochemical Impedance Data II. Transformations in the Complex Plane, J. Electrochem. Soc., 1986, 133, p 2018–2024
M. Urquidi-Macdonald, S. Real, and D.D. Macdonald, Applications of Kramers—Kronig Transforms in the Analysis of Electrochemical Impedance Data—III. Stability and Linearity, Electrochim. Acta, 1990, 35, p 1559–1566
H. Shin and F. Mansfeld, Concerning the Use of the Kramers-Kronig Transforms for the Validation of Impedance Data, Corros. Sci., 1988, 28, p 933–938
Z. Feng, X. Cheng, C. Dong, L. Xu, and X. Li, Passivity of 316L Stainless Steel in Borate Buffer Solution Studied by Mott-Schottky Analysis, Atomic Absorption Spectrometry and X-ray Photoelectron Spectroscopy, Corros. Sci., 2010, 52, p 3646–3653
G. Zhang and Y. Cheng, Corrosion of X65 Steel in CO2-Saturated Oilfield Formation Water in the Absence and Presence of Acetic Acid, Corros. Sci., 2009, 51, p 1589–1595
J. Sun, G. Zhang, W. Liu, and M. Lu, The Formation Mechanism of Corrosion Scale and Electrochemical Characteristic of Low Alloy Steel in Carbon Dioxide-Saturated Solution, Corros. Sci., 2012, 57, p 131–138
J.M. Bockris and D. Drazic, The Kinetics of Deposition and Dissolution of Iron: Effect of Alloying Impurities, Electrochim. Acta, 1962, 7, p 293–313
S. Nešić, Key Issues Related to Modelling of Internal Corrosion of Oil and Gas Pipelines—A Review, Corros. Sci., 2007, 49, p 4308–4338
Wu K-h, Zhu L-q, Li W-p, and Liu H-c, Effect of Ca2+ and Mg2+ on Corrosion and Scaling of Galvanized Steel Pipe in Simulated Geothermal Water, Corros. Sci., 2010, 52, p 2244–2249
C.-N. Cao, On the Impedance Plane Displays for Irreversible Electrode Reactions Based on the Stability Conditions of the Steady-State—I. One State Variable Besides Electrode Potential, Electrochim. Acta, 1990, 35, p 831–836
Y. Zhi-Ming and Y. Deng-Feng, A Theoretical Method to Calculate the Surface Free Energies of Crystals, Acta Phys. Sin., 2005, 54(8), p 3822–3830
L. Pauling, The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, Cornell University Press, Ithaca, 1960
J. Gray, B. El Dasher, and C. Orme, Competitive Effects of Metal Dissolution and Passivation Modulated by Surface Structure: An AFM and EBSD Study of the Corrosion of Alloy 22, Surf. Sci., 2006, 600, p 2488–2494
B.R. Kumar, R. Singh, B. Mahato, P. De, N. Bandyopadhyay, and D. Bhattacharya, Effect of Texture on Corrosion Behavior of AISI, 304L Stainless Steel, Mater. Charact., 2005, 54, p 141–147
H. Park and J. Szpunar, The Role of Texture and Morphology in Optimizing the Corrosion Resistance of Zinc-Based Electrogalvanized Coatings, Corros. Sci., 1998, 40, p 525–545
K. Ralston, N. Birbilis, and C. Davies, Revealing the Relationship Between Grain Size and Corrosion Rate of Metals, Scripta Mater., 2010, 63, p 1201–1204
Van Hunnik E, Pots B, Hendriksen E. The Formation of Protective FeCO3 Corrosion Product Layers in CO2 Corrosion, Paper No. 6. CORROSION/96, NACE International, Houston, TX, 1996
T. Minoda and H. Yoshida, Effect of Grain Boundary Characteristics on Intergranular Corrosion Resistance of 6061 Aluminum Alloy Extrusion, Metall. Mater. Trans. A., 2002, 33, p 2891–2898
H. Tanaka, H. Esaki, K. Yamada, K. Shibue, and H. Yoshida, Improvement of Mechanical Properties of 7475 Based Aluminum Alloy Sheets by Controlled Warm Rolling, Mater. Trans., 2004, 45, p 69–74
Acknowledgments
The authors are grateful to the Natural Science Foundation of Tianjin, China (13JCYBJC18200), and the authors also wish to express their thanks to the Offshore Oil Engineering Co., Ltd., China for providing the A106B steel weld joint samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Jing, H., Han, Y. et al. Effect of Welding Heat Input on the Corrosion Resistance of Carbon Steel Weld Metal. J. of Materi Eng and Perform 25, 565–576 (2016). https://doi.org/10.1007/s11665-015-1815-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-015-1815-4