Abstract
This work reports morphologically alike, high-quality monolayer MoS2 flakes with a similar strain at various growth temperatures (750–900°C) achieved by adjusting sulfur temperature. The growth dynamics of MoS2 are correlated with changes in the photoluminescence (PL) and Raman peak positions. Monolayer MoS2 crystals are synthesized at different growth temperatures from 750°C to 900°C using chemical vapor deposition (CVD). We examined the structural quality and aimed to extract the recombination mechanisms in MoS2 using low-temperature, variable, and low-laser-intensity PL measurements. Our studies of the defect-associated bound exciton emission are well correlated with the blueshift in the A1g mode of Raman spectra, blueshift in PL spectra, and x-ray photoelectron spectroscopy results for crystal grown at 900°C. Our research findings not only shed light on a thorough, non-intrusive method for modifying growth parameters to enhance optical performance, but they also suggest a way to modify the optical characteristics of MoS2 while maintaining the morphology.
Similar content being viewed by others
Introduction
Beyond graphene, the discovery of two-dimensional (2D) materials, particularly transition-metal dichalcogenides (TMDC),1,2 has widened the scope of research into 2D materials. For their potential use in low-dimensional electrical and optical devices in a range of sectors, such as optoelectronics,1,2,3,4,5,6 catalysts,7 sensors,7 memory cell8 and field effect transistors,9 monolayer TMDCs such as MoS2, WS2, and WSe2 have gained considerable attention in recent years. These layered materials differ from their bulk counterparts in several ways. The bulk equivalent of MoS2 has an indirect bandgap of 1.29 eV,10 whereas monolayer MoS2 has a direct bandgap of 1.9 eV.10 Because of the inversion symmetry breaking that occurs when a monolayer of MoS2 is separated from a bulk crystal, the bandgap shifts from indirect to direct, increasing the optical bandgap (for example, from 1.29 eV for bulk MoS2 to 1.9 eV for monolayer MoS2) due to the significantly enhanced Coulomb interaction.10 The substantial exciton- and trion-binding energies,11 inversion symmetry breaking with spin-orbital coupling,12 and valley Hall effect13 of MoS2 opens exciting potential in next-generation nanoelectronics and photonics applications.13
Through powerful spectroscopic techniques such as Raman and PL spectroscopy, electronic and vibrational properties of 2D flakes14,15 are quantified efficiently. Raman spectroscopy is a non-destructive technique that has been widely used to examine 2D material layer counts, twist angles between layers, edge qualities and kinds, and the impacts of perturbations such as strain, doping, and magnetic and electric fields. MoS2 exhibits two IR-active modes and four Raman-active modes15 (E12g, E22g, A1g, and E1g) (A2u and E1u). While the A1g mode is attributed to the out-of-plane vibration15 of just one S atom in opposite directions, the in-plane mode E12g is produced by the opposite vibration of two S atoms with respect to the Mo between them. The in-plane covalent bonding is robust in these materials, whereas the interlayer van der Waals interaction is weak. As the layer number increases, in-plane mode is believed to be affected by the long-range interlayer coulombic interaction between molybdenum atoms15 due to the increase in the dielectric tensor. On the contrary, the out-of-plane mode is found to be more sensitive to adsorbates on the MoS2 surface and electron doping.16,17 As MoS2 is reduced from a multi-layer to a monolayer, a direct bandgap excitonic transition is seen to significantly increase quantum yield. However, factors including crystal structure, local doping, and strain are likely to have an impact on the PL signal’s intensities and peak positions. Recent discoveries have shown that strain can play a vital role in modulating the bandgap of TMDCs.16,17,18,19,20,21,22,23,24,25,26 Additionally, the electrical band structure and mobility of 2D layers have been influenced by substrate-induced effects.27,28 The strain and charge doping in 2D materials shift its phonon modes, and these shifts are due to the anharmonicity of molecular potentials, which can be probed by micro-Raman spectroscopy.28 The mechanical strain28 is shown to perturb the band structure of MoS2 and carrier effective mass. Since the in-plane E12g mode is sensitive to strain,28 thus, on applying compressive or tensile strain, the E12g mode either blue or red shifts, whereas the out-of-plane mode A1g remains constant. Shifts in the E12g and A1g modes are seen in substrates such as sapphire, mica, and SiO2, which is a sign of strain and charge doping from the underlying substrates. Further, the A1g phonons with atomic displacements parallel to the c axis are involved in controlling the mobility of the carriers.29
The transport and optical characteristics of the host material are greatly influenced by electrons, holes, and excitons. Lattice point defects such as vacancies and interstitials in semiconductors can function as very effective traps for these particles.30 If excitons attached to defects combine again radiatively, light is emitted at energies below the optical band transition energy. Because of the electron wave function's more precise localization, these interactions are stronger in decreased dimensions. In the past, vacancy defects in pristine MoS2 have been introduced and controlled by electron irradiation,9,30 thermal annealing30 or ion-beam irradiation.31 The most effective approach for producing high-quality, large-scale films or crystals is chemical vapor deposition (CVD)32; however, because chalcogens are highly volatile, as-grown crystals are susceptible to sulfur deficiencies33 or anionic vacancies. These sulfur vacancies act as scattering centers and, therefore, are found to degrade the device performance by lowering the mobility. However, they act as adsorbing sites that are exploited to improve the HER catalytic performance.34 Currently, to the best of our knowledge, the growth of MoS2 is most often reported at lower growth temperature (700–800°C), while the morphology varies depending on growth temperatures. In this work, we have achieved morphologically similar, high-quality monolayer flakes with similar strain at different growth temperatures (750–900°C) by tuning the sulfur temperature. The as-grown crystals are observed to have varying sulfur vacancies. The variations in PL and Raman peak positions are correlated with the growth dynamics of MoS2. Additionally, the quality of the crystals grown at different temperatures is analyzed by low temperature and varied laser intensity PL measurements.
Experimental Section
Growth Setup
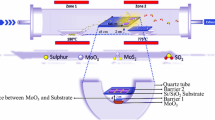
In this study, utilizing solid sulfur powder and molybdenum trioxide (MoO3) as precursors, we synthesize monolayer MoS2 films at 750°C, 800°C, 850°C, and 900°C in 20 min. With an additional S (sulfur powder) heating source upstream through a heating tape, a single-zone horizontal tube furnace (Fig. 1a) is employed to generate the monolayer MoS2 directly on SiO2/Si substrate. Approximately 200 mg of sulfur-containing quartz is added upstream, while the heating zone’s core contained SiO2/Si, Si substrates and 10 mg of MoO3 powder. SiO2/Si substrate (1.5 cm × 1 cm) is cleaned with acetone, IPA (isopropyl alcohol), and DI water before being placed on top of the MoO3 powder with a spacing of approximately 0.5 mm. To reduce the oxygen content and eliminate any remaining impurities, the tube is then purged with argon for 30 min and then pumped down to a pressure of 0.667 kPa. The heating process that corresponds to the temperature at the furnace's center begins with a slow ramping rate of between 11°C and 22°C per min, depending on the temperature set point for the chamber. This is followed by a gradual heating from room temperature to 575°C at a rate of about 30°C per min. The furnace’s temperature is maintained at 750°C, 800°C, 850°C, and 900°C for 20 min before naturally cooling to ambient temperature. Sulfur begins to slowly evaporate at a temperature near 575°C (Fig. 1b) in the furnace's center and is held at growth temperatures for the same period. The entire growing procedure is carried out with 100 sccm argon. The sulfur heating temperature is adjusted with the growth temperature to obtain similar morphology (see supplementary Table SI).
(a) Experimental setup for the chemical vapor deposition (CVD) growth of single-layer MoS2 on SiO2/Si. MoO3 is placed inside the heating zone of a furnace and sandwiched between Si and SiO2/Si, while sulfur is loaded upstream outside the furnace and heated separately. (b) Growth (orange) and sulfur (red) heating profile. (c)–(f) Optical images of MoS2 at 750°C, 800°C, 850°C and 900°C, respectively. All scale bars are 10 µm. (g) Atomic force microscopy (AFM) images of MoS2 grains.
Characterization
A Keyence VHX-6000 microscope is used to capture the MoS2 optical microscope (OM) images. Renishaw inVia Reflex Raman and PL spectroscopy are utilized to examine the structural and optical characteristics of as-grown MoS2 grains at room and low temperatures. The 532 nm line of a solid-state laser is used to excite the samples, and a 50X objective is used to focus the laser beam. For the Raman (PL) measurements, 1.57 mW of laser power and 1800 lines/m of grating are utilized, respectively. An atomic force microscope (Bruker Nano AFM) in ScanAssyst mode is used to examine the surface topography of the grains. A Thermo Scientific ESCALAB 250Xi is used to conduct x-ray photoelectron spectroscopic investigations with an Al-Ka (1486.6 eV) x-ray source.
Results and Discussion
MoO3 is first transformed to molecular clusters35 during the growth process, which is followed by condensing of these molecular clusters into MoO3−x suboxide nanoparticles under the influence of sulfur vapors at 575°C. Suboxide nanoparticles are then sulfurized to produce monolayer or few-layered MoS2.36 The dynamics of the growth process are greatly influenced by sulfur vapor concentration. The generation of suboxide or oxi-chalcogenide particles with an orthorhombic crystal structure is initiated by the weak sulfur environment.37
The strong sulfur environment stems from the formation of transition-metal fullerenes, while a moderately reducing environment results in a TMDC monolayer.36,37 Therefore, it becomes crucial to optimize the sulfur temperature in relation to the growth temperature. Experimentally, it has been found that the relatively stronger sulfur environment with respect to the growth temperature yields continuous film of MoS2 instead of triangular domains. The reaction is possibly occurred in the following steps38:
At substrate regions directly above the precursor, the vapor flux is greatest. The concentration of MoO3−x varies over the substrate’s length. Because of the high precursor ratio in the center of the substrate, there is a mixture of continuous film and large flakes of around 50 µm to 70 µm. At the downstream end of the substrate, comparatively smaller flakes of around 10–30 µm are observed (see supplementary Figure S1). The increase in growth temperature results in the increase of domain size. At lower growth temperature, fewer active mobile species are produced due to the low sublimation rate of precursors. Since the diffusion rate is low at lower growth temperatures, the grain size obtained is comparatively small. Increase in temperature accelerates the diffusion of mobile species and thus larger grains are produced. Additionally, it is discovered that when the growth temperature increases, the nucleation density decreases. Our findings are consistent with the nucleation model of the vapor phase deposition proposed by Burton and Cabrera, which states that the nucleation probability is inversely related to substrate temperature and inversely proportional to supersaturation.39 The optical mapping of CVD-grown MoS2 shows uniform flake color in Fig. 1c–f, indicating that the crystals are free from grain boundaries and adlayers. Each grain's step height is 0.91 nm (Fig. 1g), further confirming the growth of a monolayer MoS2.27
Raman and PL spectroscopy are utilized to collect lattice vibrations and excitonic (electron–hole pair generation) properties, respectively, of MoS2 grown by CVD. The Raman mapping of MoS2 grains at all growth temperatures is presented in supplementary Figure S2 and reveals that the as-grown crystals exhibit uniform crystallinity and spatial uniformity. The Raman spectra of MoS2 grains grown at different temperatures are displayed in Fig. 2a. The E12g peak which is attributed to the vibration of the Mo-S bond in the in-plane direction is found at 382.55 cm−1 for the crystals grown at different temperatures. From the Raman spectra obtained in Fig. 2a, the change in wavenumber is plotted with respect to the growth temperatures in Fig. 2b. It is shown in Fig. 2b that the A1g mode (attributed to the vibration of the Mo-S bond in the out-of-plane direction) for growth temperatures 750°C, 800°C, and 850°C (shown by the red dotted line) is found at 404.62 cm−1 while for crystals grown at 900°C, this mode is found at 407.21 cm−1, and thus it is up-shifted by 2.6 cm−1, while the E12g mode (shown by the black dotted line) remains constant at 382.55 cm−1. We observe a strong PL emission peak (Fig. 2c) around 1.81–1.83 eV for grains grown at 750°C, 800°C, and 850°C respectively, and a weaker emission peak around 1.89 eV. However, for a growth temperature of 900°C, the PL peak shows a blueshift to 1.87–1.91 eV (Fig. 2d). The two bands, A and B, have been classified as direct excitonic transitions as a result of the valence band's spin–orbit splitting. The relative strengths of the A and B emission features in our samples are a sign of a high-quality monolayer sample.40 Furthermore, the PL emission from the MoS2 grains grown at 750°C, 800°C, and 850°C is found to be enhanced around six- to sevenfold compared to the emission intensity of the MoS2 grains grown at 900°C.
(a) Raman spectra for MoS2 grains at growth temperatures 750°C, 800°C, 850°C and 900°C, respectively. (b) Change in wavenumber of the A1g mode and E12g mode with respect to growth temperatures. (c) Photoluminescence spectra for MoS2 grains at growth temperatures 750°C, 800°C, 850°C and 900°C, respectively. (d) Change in bandgap of as-grown MoS2 grains with respect to the growth temperatures. (e) Optical image of flakes after wet transfer. Scale is 100 µm. (f) Comparison of Raman spectra before and after wet transfer.
It has been well established that both strain and charge doping41,42,43 have an impact on the optical bandgap modulation of MoS2. MoS2 bandgap has been found to increase due to compressive strain (i.e., PL blueshift, E12g mode blueshift), and tensile strain decreases the bandgap (i.e., PL redshift, E12g mode redshift),44 while the A1g mode remains constant. To investigate the presence of strain, the Raman measurements are recorded before and after the wet transfer of flakes. The optical mapping in Fig. 2e shows the clean transfer of the grown flakes to the other SiO2 substrate. It is observed that after the transfer there is a blueshift of 4 cm−1 in the E12g mode (Fig. 2f), indicative of tensile strain present in our grown samples. The Raman spectra of the as-grown samples in Fig. 2a show that the E peak is constant for all growth temperatures, thus indicating that there is some strain present at all growth temperatures. As a result, the strain cannot explain the observed blueshift. To clarify the cause of the PL blueshift seen in our sample, measurements at low laser and varied intensities are performed to extract the information about the recombination mechanism and presence of defects.
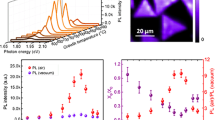
High excitation power intensities are predicted to cause the PL intensity of the bound exciton (Xb) to saturate when its associated defects are present.45 Bound excitons (Xb) are associated with the defects present in the crystals. An e–h pair bound to a defect is usually the cause of an additional energy lower than the bandgap energy (free neutral excitons, XO). Therefore, at low laser excitation, the bound exciton peak is enhanced. Defect peak intensity as a function of excitation power dependence is shown in Fig. 3a. At 900°C growth temperature, there is a defect origin peak indicating the presence of defects. The intensity of the bound exciton peak is found to be pronounced for the 900°C growth temperature. To further confirm the presence of defects and extract information on the recombination mechanism at the highest growth temperature, PL spectra are recorded at different laser intensities (Fig. 3b–h, supplementary Figure S3). The luminescence intensity I of PL emission lines is reported to be proportional to Pk, where P is the power of the exciting laser radiation and k has a value of 1 < k < 2 for band-to-band transition and k < 1 for transitions caused by defects and donors/acceptors.46 The plot of PL intensity area under the curve46 with respect to different excitation power is shown in the insets in Fig. 3b–h. It is a nearly linear plot with a positive slope, which improves linearly from 750°C to 850°C growth temperature, thus showing that the recombination is mainly direct band-to-band radiative, and then decreases (Fig. 3j) for 900°C growth temperature, implying the presence of defect states.46
(a) PL intensity of bound excitons (Xb) and free excitons (Xo) as a function of excitation laser power for crystal grown at 900°C. PL spectra at different laser excitation power as a function of photon energy for (b) crystals grown at 750°C, inset. (c) PL intensity area variation vs. different laser excitation. (d) Crystals grown at 800°C, inset. (e) PL intensity area variation vs. different laser excitation. (f) Crystals grown at 850°C, inset. (g) PL intensity area variation vs. different laser excitation. (h) Crystals grown at 900°C, inset. (i) PL intensity area variation vs. different laser excitation. (j) Slope of PL curves (obtained from linear fitting of the data points collected at different laser power) measured for all growth temperatures.
We further assess the optical quality of the as-grown MoS2 crystals by performing low-temperature PL measurements (Fig. 4a–d). The disparities in PL intensities of MoS2 grown at different temperatures are easier to understand once the defects in MoS2 have been identified. The PL quantum efficiency is as follows:
where radiative and non-radiative recombination rates are kx and knx, respectively; the non-radiative recombination caused by a defect is represented by kdefect47; the electron relaxation in the conduction and valence bands is represented by krelax; and other non-radiative recombination processes, such as Auger recombination,47 are represented by kother. As the defect concentration increases for the crystal grown at 900°C, kdefect and ultimately knx significantly result in PL intensity quenching. TMDCs grown by CVD feature many chalcogen vacancies because chalcogenides are highly volatile, thus making them extremely susceptible to chalcogen deficiencies.47 The S vacancies are found to be the most common point defects in MoS2grown by CVD, and they have the ability to introduce localized donor states with energies that were 0.3 eV below the conduction band minimum and 0.2 eV above the valence band minimum within the bandgap.30 The bandgaps of the direct-gap semiconductor monolayer MoS2 are located at the K and K′ points of the Brillouin zone. In the absence of charged impurities, short-range defects, S vacancies, or trapped charges47 at the interface between MoS2 and oxide dielectrics, the majority of excited electrons in MoS2 grains shift to the lower states. However, in a situation of localized defects (Fig. 4e), produces a continuum of states. Within a diffusion length of the edge of the defect, the trap will draw in electrons and holes, causing them to non-radiatively recombine via the continuum of states to quench the PL intensity. The photoluminescence results at low laser intensity and low temperature show that the defect level is present around 1.65 eV. Since doping typically alters the position of the Raman A1g mode, the upshift of the Raman A1g mode seen in the MoS2 crystals grown at 900°C confirms this claim.
Furthermore, an investigation employing X-ray photoelectron spectroscopy is used to investigate the surface chemical states of Mo and S in our morphologically identical MoS2 crystals grown at various temperatures to substantiate the findings of sulfur vacancies. Further, the results in Fig. 5 show that Mo is present in two distinct chemical environments across all samples. In as-grown MoS2 crystals, Mo is found to exist in two different oxidation states, Mo4+ and Mo6+, which correspond to the Mo-S and Mo-O bonding conditions.48,49 The Mo:S ratio is found to be approximately 1:1.83 ± 0.13 (750°C), 1:1.8056 ± 0.31 (800°C), 1:1.1.922 ± 0.32 (850°C), and 1:1.6173 ± 0.13 (900°C). This indicates the change in the stoichiometry of MoS2 crystals grown at different temperatures. The amount of sulfur precursor placed in the CVD growth tube is the same at each growth temperature, but the variation in the stoichiometry of MoS2 suggests that the growth temperature played a role in the Mo:S ratio. According to the findings, MoS2 crystals grown at 900°C are found to have more anionic vacancies than crystals grown at other temperatures. At the higher growth temperatures, the results indicate that extra charges are introduced by the sulfur vacancies and the extra sulfur screens the formation of excitons. It has been found that Mo dz2 states contribute to the conduction band minimum (CBM) and valence band maximum (VBM) at the K point, while Mo dz2 and S p states dominate the CBM at K-Γ and the VBM at Γ.50 It is possible that the change in the sulfur ratio affected the VBM at Γ, thus quenching the PL efficiency. Fewer anionic vacancies are created in our case than in other techniques such as thermal annealing or electron beam irradiation because the intensity of bound excitons (Xb) is lower.
Conclusions
The growth of MoS2 at different temperatures while maintaining similar morphology is successfully demonstrated. To explore the structural defects in MoS2produced by CVD, low-temperature, variable laser intensity, and low laser intensity PL measurements are carried out. Our experimental results suggest that crystals grown at higher temperature (900°C) are prone to sulfur vacancies. The Mo:S ratio determined by XPS shows lower sulfur concentration in crystals produced at 900°C. The growth temperature of 850°C exhibits higher quality crystals. Experimental evidence shows that the anionic vacancies act as defect sites to modulate MoS2 optical bandgap in a way that causes a new peak to appear below it. These bound excitons are produced as a result of localizing excitons at the defect sites. Our work provides an effective way to grow these crystals at higher temperatures and achieve tunable electronic and optical properties of MoS2, and also provides a path to grow different TMDC layers and their heterostructures at higher growth temperatures.
References
K.S. Novoselov, D. Jiang, F. Schedin, T.J. Booth, V.V. Khotkevich, S.V. Morozov, and A.K. Geim, Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. 102(30), 10451 (2005).
S. Bertolazzi, J. Brivio, and A. Kis, Stretching and breaking of ultrathin MoS2. ACS Nano 5(12), 9703 (2011).
R. Fivaz and E. Mooser, Electron-phonon interaction in semiconducting layer structures. Phys. Rev. 136(3A), A833 (1964).
R. Saito, Y. Tatsumi, S. Huang, X. Ling, and M.S. Dresselhaus, Raman spectroscopy of transition metal dichalcogenides. J. Phys. Condens. Matter 28(35), 353002 (2016).
O. Lopez-Sanchez, D. Lembke, M. Kayci, A. Radenovic, and A. Kis, Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 8, 497 (2013).
Q.H. Wang, K. Kalantar-Zadeh, A. Kis, J.N. Coleman, and M.S. Strano, Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7(11), 699 (2012).
S.Z. Butler, S.M. Hollen, L. Cao, Y. Cui, J.A. Gupta, H.R. Gutiérrez, T.F. Heinz, S.S. Hong, J. Huang, A.F. Ismach, and E. Johnston-Halperin, Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 7(4), 2898 (2013).
S. Bertolazzi, D. Krasnozhon, and A. Kis, Nonvolatile memory cells based on MoS2/graphene heterostructures. ACS Nano 7(4), 3246 (2013).
T. Shen, F. Li, L. Xu, Z. Zhang, F. Qiu, Z. Li, and J. Qi, High mobility monolayer MoS2 transistors and its charge transport behavior under E-beam irradiation. J. Mater. Sci. 55(29), 14315 (2020).
R. Ganatra and Q. Zhang, Few-layer MoS2: a promising layered semiconductor. ACS Nano 8(5), 4074 (2014).
D.Y. Qiu, H. Felipe, and S.G. Louie, Optical spectrum of MoS2: many-body effects and diversity of exciton states. Phys. Rev. Lett. 111(21), 216805 (2013).
D. Xiao, G.B. Liu, W. Feng, X. Xu, and W. Yao, Coupled spin and valley physics in monolayers of MoS2 and other group-VI dichalcogenides. Phys. Rev. Lett. 108(19), 196802 (2012).
K.F. Mak, K.L. McGill, J. Park, and P.L. McEuen, The valley Hall effect in MoS2 transistors. Science 344(6191), 1489 (2014).
Y. Wang, C. Cong, C. Qiu, and T. Yu, Raman spectroscopy study of lattice vibration and crystallographic orientation of monolayer MoS2 under uniaxial strain. Small 9(17), 2857 (2013).
X. Zhang, X.F. Qiao, W. Shi, J.B. Wu, D.S. Jiang, and P.H. Tan, Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material. Chem. Soc. Rev. 44(9), 2757 (2015).
K. He, C. Poole, K.F. Mak, and J. Shan, Experimental demonstration of continuous electronic structure tuning via strain in atomically thin MoS2. Nano Lett. 13(6), 2931 (2013).
Z. Li, Y. Lv, L. Ren, J. Li, L. Kong, Y. Zeng, Q. Tao, R. Wu, H. Ma, B. Zhao, and D. Wang, Efficient strain modulation of 2D materials via polymer encapsulation. Nat. Commun. 11(1), 1 (2020).
T. Li, Ideal strength and phonon instability in single-layer MoS2. Phys. Rev. B 85(23), 235407 (2012).
M. Hosseini, M. Elahi, M. Pourfath, and D. Esseni, Strain induced mobility modulation in single-layer MoS2. J. Phys. D Appl. Phys. 48(37), 375104 (2015).
S. Yu, H.D. Xiong, K. Eshun, H. Yuan, and Q. Li, Phase transition, effective mass and carrier mobility of MoS2 monolayer under tensile strain. Appl. Surf. Sci. 325, 27 (2015).
X. Xu, C. Wang, W. Xiong, Y. Liu, D. Yang, X. Zhang, and J. Xu, Strain regulated interlayer coupling in WSe2/WS2 heterobilayer. Nanotechnology 33(8), 085705 (2021).
H.J. Conley, B. Wang, J.I. Ziegler, R.F. Haglund Jr., S.T. Pantelides, and K.I. Bolotin, Bandgap engineering of strained monolayer and bilayer MoS2. Nano Lett. 13(8), 3626 (2013).
R. Sahu, D. Radhakrishnan, B. Vishal, D.S. Negi, A. Sil, C. Narayana, and R. Datta, Substrate induced tuning of compressive strain and phonon modes in large area MoS2 and WS2 van der Waals epitaxial thin films. J. Cryst. Growth 470, 51 (2017).
E. Scalise, M. Houssa, G. Pourtois, V. Afanas’ev, and A. Stesmans, Strain-induced semiconductor to metal transition in the two-dimensional honeycomb structure of MoS2. Nano Res. 5, 43 (2012).
M. Ghorbani-Asl, S. Borini, A. Kuc, and T. Heine, Strain-dependent modulation of conductivity in single-layer transition-metal dichalcogenides. Phys. Rev. B 87(23), 235434 (2013).
S.B. Desai, G. Seol, J.S. Kang, H. Fang, C. Battaglia, R. Kapadia, J.W. Ager, J. Guo, and A. Javey, Strain-induced indirect to direct bandgap transition in multilayer WSe2. Nano Lett. 14(8), 4592 (2014).
W.H. Chae, J.D. Cain, E.D. Hanson, A.A. Murthy, and V.P. Dravid, Substrate-induced strain and charge doping in CVD-grown monolayer MoS2. Appl. Phys. Lett. 111(14), 143106 (2017).
A. Koma, Van der Waals epitaxy—a new epitaxial growth method for a highly lattice-mismatched system. Thin Solid Films 216(1), 72 (1992).
B. Chakraborty, A. Bera, D.V.S. Muthu, S. Bhowmick, U.V. Waghmare, and A.K. Sood, Symmetry-dependent phonon renormalization in monolayer MoS2 transistor. Phys. Rev. B 85(16), 161403 (2012).
S. Tongay, J. Suh, C. Ataca, W. Fan, A. Luce, J.S. Kang, J. Liu, C. Ko, R. Raghunathanan, J. Zhou, and F. Ogletree, Defects activated photoluminescence in two-dimensional semiconductors: interplay between bound, charged and free excitons. Sci. Rep. 3(1), 1 (2013).
A. Inoue, T. Komori, and K.I. Shudo, Atomic-scale structures and electronic states of defects on Ar+−ion irradiated MoS2. J. Electron Spectrosc. Relat. Phenom. 189, 11 (2013).
Y. Gong, G. Ye, S. Lei, G. Shi, Y. He, J. Lin, X. Zhang, R. Vajtai, S.T. Pantelides, W. Zhou, and B. Li, Synthesis of millimeter-scale transition metal dichalcogenides single crystals. Adv. Func. Mater. 26(12), 2009 (2016).
S. Barja, S. Refaely-Abramson, B. Schuler, D.Y. Qiu, A. Pulkin, S. Wickenburg, H. Ryu, M.M. Ugeda, C. Kastl, C. Chen, C. Hwang, A. Schwartzberg, S. Aloni, S.K. Mo, D. Frank Ogletree, M.F. Crommie, O.V. Yazyev, S.G. Louie, J.B. Neaton, and A. Weber-Bargioni, Identifying substitutional oxygen as a prolific point defect in monolayer transition metal dichalcogenides. Nat. Commun. 10(1), 3382 (2019).
S. Geng, W. Yang, Y. Liu, and Y. Yu, Engineering sulfur vacancies in basal plane of MoS2 for enhanced hydrogen evolution reaction. J. Catal. 391, 91 (2020).
A. Zak, Y. Feldman, V. Alperovich, R. Rosentsveig, and R. Tenne, Growth mechanism of MoS2 fullerene-like nanoparticles by gas-phase synthesis. J. Am. Chem. Soc. 122(45), 11108 (2000).
J.D. Cain, F. Shi, J. Wu, and V.P. Dravid, Growth mechanism of transition metal dichalcogenide monolayers: the role of self-seeding fullerene nuclei. ACS Nano 10(5), 5440 (2016).
Y. Feldman, E. Wasserman, D.J. Srolovitz, and R. Tenne, High-rate, gas-phase growth of MoS2 nested inorganic fullerenes and nanotubes. Science 267(5195), 222 (1995).
S. Kataria, S. Wagner, T. Cusati, A. Fortunelli, G. Iannaccone, H. Pandey, G. Fiori, and M.C. Lemme, Growth-induced strain in chemical vapor deposited monolayer MoS2: experimental and theoretical investigation. Adv. Mater. Interfaces 4(17), 1700031 (2017).
W.K. Burton and N. Cabrera, Crystal growth and surface structure. Part I. Discuss. Faraday Soc. 5, 33 (1949).
K.M. McCreary, A.T. Hanbicki, S.V. Sivaram, and B.T. Jonker, A-and B-exciton photoluminescence intensity ratio as a measure of sample quality for transition metal dichalcogenide monolayers. APL Mater. 6(11), 111106 (2018).
M. Velicky, A. Rodriguez, M. Bousa, A.V. Krayev, M. Vondracek, J. Honolka, and O. Frank, Strain and charge doping fingerprints of the strong interaction between monolayer MoS2 and gold. J. Phys. Chem. Lett. 11(15), 6112 (2020).
A. Michail, N. Delikoukos, J. Parthenios, C. Galiotis, and K. Papagelis, Optical detection of strain and doping inhomogeneities in single layer MoS2. Appl. Phys. Lett. 108, 173102 (2016).
H. Kim, T. Lee, H. Ko, S.M. Kim, and H. Rho, Influence of chemical treatment on strain and charge doping in vertically stacked monolayer–bilayer MoS2. Appl. Phys. Lett. 117, 202104 (2020).
K. Ponnusamy, S. Durairaj, and S. Chandramohan, Effect of growth temperature on the morphology control and optical behavior of monolayer MoS2 on SiO2 substrate. J. Mater. Sci. Mater. Electron. 33(12), 9549 (2022).
T.L.K.Z. Schmidt, K. Lischka, and W. Zulehner, Excitation-power dependence of the near-band-edge photoluminescence of semiconductors. Phys. Rev. B 45(16), 8989 (1992).
S. Poduri, M. Dutta, and M. Stroscio, Characterization of CdS nanowires self-assembled in a nanoporous alumina template. J. Electr. Mater. 43, 3979 (2014).
A. Zafar, H. Nan, Z. Zafar, Z. Wu, J. Jiang, Y. You, and Z. Ni, Probing the intrinsic optical quality of CVD grown MoS2. Nano Res. 10(5), 1608 (2017).
X. Wei, Z. Yu, F. Hu, Y. Cheng, L. Yu, X. Wang, M. Xiao, J. Wang, X. Wang, and Y. Shi, Mo-O bond doping and related-defect assisted enhancement of photoluminescence in monolayer MoS2. AIP Adv. 4(12), 123004 (2014).
S. Durairaj, K. Ponnusamy, N.B. Shinde, S.K. Eswaran, V. Asokan, J.B. Park, and S. Chandramohan, Oxygen-driven growth regulation and defect passivation in chemical vapor deposited MoS2 monolayers. Cryst. Growth Des. 21(12), 6793 (2021).
F. Wang, J. Wang, S. Guo, J. Zhang, Z. Hu, and J. Chu, Tuning coupling behavior of stacked heterostructures based on MoS2, WS2, and WSe2. Sci. Rep. 7(1), 1 (2017).
Acknowledgments
The authors gratefully acknowledge the financial support from AFOSR grant No. FA9550-19-1-0282. The authors would also like to thank Electron Microscopy Core (EMC) for Raman and photoluminescence (PL) measurements, Nanotechnology Core Facility (NCF) for atomic force microscopy (AFM) measurements, and Northwestern University Atomic and Nanoscale Characterization Equipment Center for x-ray photoelectron spectroscopy (XPS) measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomar, R., Hsu, B., Perez, A. et al. Probing Sulfur Vacancies in CVD-Grown Monolayer MoS2 on SiO2/Si in the Temperature Range 750–900°C. J. Electron. Mater. 52, 5513–5520 (2023). https://doi.org/10.1007/s11664-023-10463-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10463-1