Abstract
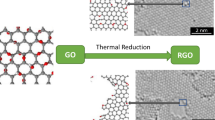
Free-standing reduced graphene oxide (rGO) papers were prepared by chemical reduction, thermal reduction and combined chemical-thermal reduction, respectively. Four-point probe and nanoindentation experiments were conducted to investigate the electrical and mechanical properties of rGO papers. The rGO paper prepared by soaking in L-ascorbic acid and thermal annealing in argon at 1000 °C (labeled rGO-AsA-T) showed superior electrical and mechanical properties when compared with rGO papers prepared merely by chemical reduction or thermal reduction. The as-prepared rGO-AsA-T paper exhibited an electrical conductivity of 5.7 × 104 S/m, showing an increase of 90% compared to that in the thermally annealed rGO paper and nearly 40 times that of rGO paper reduced by L-ascorbic acid. It was also found that the rGO-AsA-T paper had the lowest elastic modulus of 288 MPa, showing enhanced flexibility. The nearly free voids in rGO-AsA-T paper proved by scanning electron microscopy (SEM) were due to the capillary action in chemical reduction and were significant in improving the electrical conductivity and flexibility of the paper. The rGO-AsA-T paper with high conductivity and flexibility has a promising application in flexible electronics.





Similar content being viewed by others
References
T. Wu, Y. Xu, H. Wang, Z. Sun, and L. Zou, Carbon 171, 639 (2021).
D.G. Papageorgiou, I.A. Kinloch, and R.J. Young, Prog. Mater. Sci. 90, 75 (2017).
A. Ambrosetti, and P.L. Silvestrelli, J. Phys. Chem. Lett. 11, 2737 (2020).
Q.Q. Xiong, and Z.G. Ji, J. Alloys Compd 673, 215 (2016).
Z.F. Zhao, X.J. Teng, Q.Q. Xiong, H.Z. Chi, Y.J. Yuan, H.Y. Qin, and Z.G. Ji, Sustain. Mater. Technol 29, e00313 (2021).
Y.W. Zhang, Y.K. Wu, W. Zhong, F.Y. Xiao, M.K. Aslam, X. Zhang, and M.W. Xu, Chemsuschem 14, 1336 (2021).
Y.Y. Shi, G.J. Liu, R.C. Jin, H. Xu, Q.Y. Wang, and S.M. Gao, Carbon Energy 1, 253 (2019).
Y. Lin, G. Hou, S. Bi, X. Su, and H. Li, Funct. Mater Lett. 11, 2051024 (2020).
D.A. Dikin, S. Stankovich, E.J. Zimney, R.D. Piner, and R.S. Ruoff, Nature 448, 457 (2015).
M. Wang, L.D. Duong, J.S. Oh, N.T. Mai, S. Kim, S. Hong, T. Hwang, Y. Lee, and J.D. Nam, ACS Appl. Mater. Interfaces 6, 1747 (2014).
P. Kumar, U.N. Maiti, K.E. Lee, and S.O. Kim, Carbon 80, 453 (2014).
Y.W. Zhu, S. Murali, W.W. Cai, X.S. Li, J.W. Suk, J.R. Potts, and R.S. Ruoff, Adv. Mater. 22, 906 (2010).
H.X. Wang, and Y. Cui, Carbon Energy 1, 13 (2019).
H.F. Lu, J. Zhang, J. Luo, W.B. Gong, C.W. Li, Q.L. Li, K. Zhang, and M. Hu, Compos. Pt A-Appl. Sci. Manuf. 102, 1 (2017).
L. Peng, Z. Xu, Z. Liu, Y. Guo, P. Li, and C. Gao, Adv. Mater. 29, 1700589 (2017).
L. Dong, C. Hu, L. Song, X. Huang, N. Chen, and L. Qu, Adv. Funct. Mater. 26, 1470 (2016).
G.Q. Chen, B.H. Yuan, Y.Y. Zhan, H.M. Dai, S. He, and X.F. Chen, Mater. Chem. Phys. 252, 123292 (2020).
J.D. Renteria, S. Ramirez, H. Malekpour, B. Alonso, A. Centeno, A. Zurutuza, A.I. Cocemasov, D.L. Nika, and A.A. Balandin, Adv. Funct. Mater. 25, 4664 (2015).
S. Pei, J. Zhao, J. Du, W. Ren, and H.M. Cheng, Carbon 48, 4466 (2010).
S.H. Shen, R.F. Zhou, Y.H. Li, B. Liu, G.X. Pan, Q. Liu, Q.Q. Xiong, X.L. Wang, X.H. Xia, and J.P. Tu, Small Methods 3, 1900596 (2019).
X. Chen, L. Wei, L. Da, H. Ming, X. Wu, H. Yuan, H.L. Sun, C. Xiong, and R.S. Ruoff, ACS Nano 11, 665 (2017).
T. Kuila, S. Bose, A.K. Mishra, P. Khanra, N.H. Kim, and J.H. Lee, Prog. Mater. Sci. 57, 1061 (2012).
A. Mehmood, N.M. Mubarak, M. Khalid, R. Walvekar, and S. Mazari, J. Environ. Chem. Eng. 8, 103743 (2020).
P. Goli, H. Ning, X. Li, C.Y. Lu, and A.A. Balandin, Nano Lett. 14, 1497 (2014).
J. Ding, H. Zhao, Q. Wang, H. Dou, H. Chen, and H. Yu, Nanoscale 9, 16871 (2017).
X. Lin, X. Shen, Q. Zheng, N. Yousefi, L. Ye, Y.W. Mai, and J.K. Kim, ACS Nano 6, 10708 (2012).
C. Vallés, J.D. Nú Ez, and A.M. Benito, Carbon 50, 835 (2012).
X.J. Chen, D.L. Meng, B. Wang, B.W. Li, W. Li, C.W. Bielawski, and R.S. Ruoff, Carbon 101, 71 (2016).
Y. Zhu, S. Murali, M.D. Stoller, K.J. Ganesh, and R.S. Ruoff, Science 332, 1537 (2011).
W.S. Hummers, and R.E. Offeman, J. Am. Chem. Soc. 80, 1339 (1958).
W.C. Oliver, and G.M. Pharr, J. Mater. Res. 7, 1564 (1992).
T.D. Le, G.N. Yong, and H. Seo, Sens. Actuator B-Chem 334, 129676 (2021).
Y. Zhang, X.H. Xia, B. Liu, S.J. Deng, D. Xie, Q. Liu, Y.D. Wang, J.B. Wu, X.L. Wang, and J.P. Tu, Adv. Energy Mater 9, 1803342 (2019).
J. Zhang, H. Yang, G. Shen, C. Ping, and S. Guo, Chem. Commun. 46, 1112 (2010).
X.J. Chen, X.M. Deng, N. Yeon, Y. Huang, L. Peng, M. Huang, X. Zhang, X. Chen, D. Luo, B. Wang, X.Z. Wu, Y.F. Ma, Z. Lee, R.S. Ruoff et al., Carbon 132, 294 (2018).
A.R. Ranjbartoreh, B. Wang, X.P. Shen, and G.X. Wang, J. Appl. Phys. 109, 666 (2011).
L.Q. Tao, K.N. Zhang, H. Tian, Y. Liu, D.Y. Wang, Y.Q. Chen, Y. Yang, and T.L. Ren, ACS Nano 11, 8790 (2017).
P. Li, L. Zhao, Z. Jiang, M. Yu, and Y. Zhao, Sci. Rep. 9, 1 (2019).
Y. Zhang, and C. Pan, Diam Relat. Mater. 24, 1 (2012).
Acknowledgements
This work has been financially supported by the National Natural Science Foundation of China (61774084), by the Priority Academic Program Development of Jiangsu Higher Education Institutions, by the special fund of Jiangsu Province for the transformation of scientific and technological achievements (BA2019047), the open project of Key Laboratory of Materials Preparation and Protection for Harsh Environment, Ministry of Industry and Information Technology (XCA20013-3) and by funding of Jiangsu Innovation Program for Graduate Education (KYCX19_0175).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Shen, H., Yang, J. et al. Enhanced Conductivity and Flexibility in Reduced Graphene Oxide Paper by Combined Chemical-Thermal Reduction. J. Electron. Mater. 50, 6991–6999 (2021). https://doi.org/10.1007/s11664-021-09201-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09201-2