Abstract
Optoelectronics is an active area of research and, for few decades, development of different semiconducting materials with a wide emission window has attracted researchers. Organic light emitting diodes (OLEDs) are primarily utilized in displays and light sources that greatly contribute towards the conservation of energy and do not need a backlight for displays. Development in device efficiency, lifetime and stability is now a major concern in this particular application, and designing efficient material for OLEDs has been an active field of research for decades. Metal-organic compounds possess different optical and electronic properties due to metal and organic ligand interactions which are primarily used in OLEDs. This review is mainly focused on the Schiff bases and their metal chelates as a pure emitting layer or as a dopant material for the fabrication of R/G/B/white emitting devices. Moreover, future prospects to explore further to advance research in the OLED arena are also discussed.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In this modern era of smart devices, conservation of energy and production of clean energy are the two most challenging needs that demand significant attention, and the fabrication of devices that consume less energy is one of the ways to address these issues.1,2,3,4,5,6,7,8,9,10,11,12 LEDs with various associated advantages such as long life, bright and intense emission, wide color range, low heat radiation, instantaneous and directional lighting have grabbed great attention over the last decade due to their diverse and valuable applications.13,14,15 Although many semiconducting inorganic compounds (gallium arsenide phosphide, gallium arsenide, and indium phosphide) are being used to manufacture LEDs,16 they are crystalline and rigid, rare and expensive to process, need highly pure material that demand controlled conditions for accurate processing and are potentially toxic,17 In this context, organic fluorescent molecules have attractive prospects as optoelectronic materials as they are flexible because their chemical, optical and electronic properties can be easily altered by modifying their structure.18 Band gap tuning in these fluorescent molecules through different synthetic routes and subsequent designing of prototype organic light-emitting devices (OLEDs) have garnered immense research attention.19 OLEDs function without a backlight and emit light from their pixels, unlike liquid crystal displays (LCDs), which need a high-intensity backlight. OLEDs have high light output and are readable in sunlight with low power drain and are hence favored in portable applications. Presently, these OLEDs are used in car radios, phone displays, portable digital media players, and digital cameras.20,21
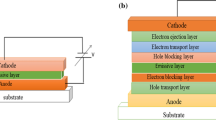
An OLED is a p-n junction of semiconducting materials with different supporting layers that produce electroluminescence (EL). The device configuration includes a substrate layer made of glass, and a transparent plastic foil to support the whole device. The emissive layer (EML) receives electrons from the cathode, while the anode removes electrons from the conducting layer, allowing holes to form. Semiconducting emissive organic layers are generally polymers or small molecules, while the conducting layer usually consists of polymers such as polyaniline, PEDOT:PSS (poly(3,4-ethylenedioxythiophene):polystyrene sulfonate), and N,N’-Bis(3-methylphenyl)-N,N’-diphenyl-1,1’-biphenyl-4,4’-diamine (TPD) which transport the holes from the anode. Charged particles such as holes and electrons move to the EML to produce EL. Tang and Van Slyke in 1987 discovered OLEDs based on polymeric material as the emitting source,22 and over the past few decades, a large number of studies have reported on the organic molecules employed in the design and fabrication of these devices. Adachi et al. reported a three-layered system wherein the host was sandwiched between the electron and hole transport layers.23,24 Later, five-layered devices were fabricated introducing a hole and electron injection layer and incorporating dopants such as rubrene and coumarin derivatives to the host layer.25 Phosphorescent dyes that participate in both triplet and singlet excited states that could potentially result in OLEDs with a 100% internal quantum yield (QY) have also been reported.26 Strategic architecture of desirable highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels in the active emitting layer is important for the rapid transfer of electrons and holes to the EML.27,28,29,30,31 Functional metal-organic materials are an evolving class of luminophores as they include the benefits of both organic dyes, which are color-tunable with strong emission and transition-metal-based emitters with large Stokes shifts and high photostability.32 These coordination complexes of organic molecules have been widely investigated for the development of electroluminescent devices that exhibit intense and bright fluorescence emission spanning the entire visible region from violet to red with high quantum efficiencies. The present article is structured to introduce the recent advances in employing Schiff bases and their complexes either as emissive materials or as dopant materials for the fabrication of R/G/B/white emitting OLED devices. The article concludes with future prospects that can be explored to further enhance research in this area for achieving devices with improved features.
Schiff Bases and Their Complexes
Schiff bases and their complexes with transition metals have been extensively explored in the pursuit of their applications in various areas including nonlinear optics, molecular/metal ion sensing, dye-sensitized solar cells, molecular magnetism and photoluminescence (PL).33,34,35,36 This group of organic compounds was reported by Hugo Schiff (1834-1915) and was named Schiff bases after him.37 The condensation reaction between the carbonyl groups and primary amines produces an azomethine group (–N=C–) containing Schiff bases. Later several efforts were described to synthesize these functional materials by employing solvent-free/clay/microwave irradiation, solvent-free/CaO/microwave, solvent-free/NaHSO4.SiO2/microwave, K-10/microwave, solvent-free/infrared irradiation, solid-state synthesis, water suspension medium, [bmim]BF4/molecular sieves, and silica/ultrasound irradiation.21,38,39,40,41,42,43,44,45 The general nucleophile addition mechanism of formation of Schiff bases is depicted in Fig. 1.46 Initially, the amine nucleophile reacts with the aldehyde or ketone to form carbinolamine which is an unstable addition product, and later undergoes acid-catalyzed dehydration to generate the imines.21
Schiff bases can act as ligands and chelate with metals by using imine nitrogen and the functional groups which are commonly associated with aldehyde counterparts.47 Heavy metal coordination enhances spin-orbit coupling by maximizing the intersystem crossing (ISC) from singlet to triplet states, and thereby the rate of radiative decay from triplet states.48 Generally, the Schiff base-metal complexes exhibit a planar structure, which has often yielded a higher quantum emission than free ligands. Schiff bases as well as their complexes are broadly utilized in constructing OLEDs to tune the emission colour and also to increase the efficiency of the device. The physical, photophysical, and photochemical properties of these complexes can be conveniently modified, and increased ligand rigidity results in complexes with lower non-radiative decay involvement, resulting in more effective devices.25,49,50,51,52 Since they are simple to acquire and can be modified in a variety of ways, Schiff bases and their metal chelates have been extensively developed. Various practical applications are based on the molecular design of different long-lived and emissive transition metal complexes.
Schiff Bases and Their Complexes As Emissive and Guest Materials
The two most important criteria for high-performance OLEDs are high carrier mobility and intense luminescence, which are achieved by planar molecules with an extended π-conjugated system.53 High-performance OLED fabrication involves various phosphorescent d6 and d8 metal complexes associated with nitrogen and/or carbon as donor atoms.54,55 Pt(II) Schiff base complexes are well known dopants with high environmental stability.25,52 Platinum (Pt) metal and the planar structure of the ligand facilitate aggregation and suppress emission quenching by encouraging intermolecular ligand-ligand and/or metal-metal interactions.56 Pt(II)-based OLED performance primarily depends on the dopant concentration, and the optimized concentration of dopant is below 5%.57,58,59,60 Sano and coworkers have reported various bidentate and tetradentate Schiff base-zinc complexes61,62 that were used in the fabrication of blue and green OLEDs. Zinc and nickel complexes of Schiff base complexes with conjugated polymer were recently reported as an active material.62,63,64 Boron (B) is a strong electrophile that has a tendency to occupy empty orbitals and attain an octet structure, forming tetra-coordinate organoboron compounds with Schiff base ligands with intense fluorescence emission.65 For the past few decades, luminescent Schiff base ligands and their metal complexes have been explored for fabricating OLEDs, with varying color emissions spanning the visible spectrum, and the following section illustrates the recent progress in this direction.
Blue Emitters
Mohan et al. synthesized four highly fluorescent Schiff bases 1a-d by condensing 4,5-dimethyl-1,2-phenylenediamine with different aldehydes such as N-ethyl-3-carboxaldehyde, fluorene-2-carboxaldehyde, N-(4-formylphenyl) carbazole, and 8-hydroxyjulolidine-9-carboxaldehyde.66 Solid-state emission with various fluorescent colors was accomplished by merely varying the peripheral group attached to the core. The HOMO and LUMO energy level values for 1d (λab = 349, 363 nm; λem = 453 nm) with the donor-acceptor feature were − 5.7 eV and − 3.2 eV, respectively, with a band gap of 2.5 eV. The device with configuration ITO (indium tin oxide)|TPD|1d|CBP|Alq3|LiF|Al, wherein TPD acted as hole injection and hole transport material, 4,4’-N,N’-dicarbazolylbiphenyl (CBP) served as hole blocking material, Tris-(8-hydroxyquinoline)aluminum (Alq3) functioned as electron transport material, aluminium (Al) as the cathode, and lithium fluoride (LiF) for improved electron injection into Alq3 was fabricated to generate bluish-green light with maximum current efficiency (CE) of 2 cd/A, a maximum brightness of 280 cd/m2 at 34 V and a power efficiency (PE) of 0.18 lm/W (Fig. 2). The structural framework of 1d incorporated a carbazole unit that offered good film-forming ability, thermal stability, charge injection and hole transport feature with enhanced and efficient EL that are desirable for optical applications.
Blue-emitting Schiff base complexes with N-substituted and carbazole carrying heteroaromatics and their device architectures (data from Ref. 66).
Zhang et al. synthesized Schiff base complexes with Pt and utilized them for phosphorescent light-emitting diode application.67 (Fig. 3) The condensation between substituted salicylaldehydes and aniline generated one-armed Schiff base ligands, which were further complexed with Pt using potassium tetrachloroplatinate (K2PtCl4) to form 2a-f, with ligand-to-ligand charge transfer (LLCT) and metal-to-ligand charge transfer (MLCT) properties. The presence of strong electron-donating or electron-withdrawing groups shifted the emission wavelength to red region, and the electrons transferred to metal complex from phenolate and imino group when they were excited from ground to excited state. The thermally stable complexes displayed band gaps in the range of 2.05–2.54 eV. Among the phosphorescent devices that were fabricated using 2b (λab = 343, 487 nm; λem = 527, 618 nm), 2c (λab = 267, 313, 386, 446 nm; λem = 613 nm), 2e (λab = 337, 451, 497 nm; λem = 565, 609 nm) and 2f (λab = 299, 442 nm; λem = 566, 606 nm) with device configuration ITO|MoO3|NPB(N,N’-bis(1-naphthyl)-N,N’-diphenyl-1,1’-biphenyl-4,4’-diamine)|mCP(1,3-bis-(N-carbazolyl)benzene)|mCP:3wt.%2a-f|TPBI(1,3,5-Tris(1-phenyl-1H-benzo[d]imidazol-2-yl)benzene)|LiF|Al, the devices with 2c and 2e emitted in the blue region, 2b emitted orange light and 2f emitted green light. The device efficiency was optimized by varying NPB, and the blue OLED doped with 3wt % 2c (80 nm) displayed a turn-on voltage of 6.3 V, a maximum brightness of 1521 cd m−2 at 17.4 V, a peak external quantum efficiency (EQE) of 0.54%, a peak luminance efficiency of 1.12 cd A−1 and a peak PE of 0.62 lm W−1.
Blue emitting Pt(II)-based one-armed complexes for phosphorescent OLEDs (data from Ref. 67).
Gusev et al. prepared a Schiff base by refluxing 4-methyl-1-phenyl-4-formylpyrazol-5-one with 1,2-ethylenediamine in ethanol, and added zinc acetate dehydrate to the reaction mixture to obtain azomethine-zinc complex 3 (λab = 336 nm; λem = 419, 433, 468 nm) with high thermal stability.68 (Fig. 4a) As the d-atomic orbital of zinc (Zn) has no significant effect on HOMO and LUMO transitions, it does not act as heavy-atom to induce spin-orbit coupling effect on the singlet-triplet ISC. The structurally rigid complex suppressed the non-radiative channels, and the unhydrated sample showed better blue emission with 70.4% quantum yield (QY). An undoped device with configuration ITO|TPD|3|PBD((2-4-biphenylyl)-5-phenyl-oxadiazole)|Ca|Al, and further, an mCP-doped device with configuration ITO|TPD|20% 3|mCP|PBD|Ca|Al to avoid concentration quenching and improve efficiency was fabricated. Both the devices showed blue emission in the range 480-490 nm with color coordinates (0.24, 0.27) and (0.22, 0.19) for undoped and doped devices, respectively. The turn on voltage was reduced due to the doping effect. The devices displayed maximum brightness of 13,000 cd m−2 at 10 V and 17,000 cd m−2 at 14 V with maximum CE of 8.6 and 13.8 cd A−1 for undoped and doped devices, respectively. The higher efficiency can be explained through exciton recombination and efficient Förster energy transfer to complex from the host.
Yet another Schiff base-Zn complex 4 which emitted blue light was synthesized by Kang et al. through a hydrolysis-free solution-based method.69 (Fig. 4b) Thin films were prepared by dispersing the metal-organic complex 4 in polymethylmethacrylate (PMMA) and cellulose acetate butyrate (CAB) polymer matrices. These metal complex-polymer hybrid films exhibited enhanced quantum efficiency (85.8% for PMMA and 30.0% for CAB) and scalability for large area fabrication, retaining the unique features of the host polymers such as good photostability, biocompatibility, transparency, and thermal and photochemical stability. The OLED device fabricated using the CAB hybrid thin films displayed luminance efficiency of 43.2% with excellent photostability. A blue-emitting Zn complex 5 in which the ligand was obtained via the condensation reaction of o-phenylenediamine and 2-hydroxy-1-naphthaldehyde was synthesized by Gondia et al.70 (Fig. 4b). The complex with an energy band gap of 3.98 eV emitted violet light with CIE (Commission International de l’Eclairage) coordinates (0.239, 0.159), which can be used as a wide band gap active material for doping with the host organic EML to obtain violet OLEDs with improved device efficiency. Cyan blue-emitting tetranuclear cyanide-bridged Mn(III)–Fe(III) complex 6 was synthesized by Donmez et al.71 (Fig. 4b). Though the ligand alone showed maximum emission in the orange region due to intra-ligand charge transfer (ILCT), the polymetallic complex exhibited cyan-blue color due to the linkage of a metal atom to the ligand and could be used as a functional material in OLEDs.
Despite steady development in luminescent materials with shorter π-conjugation that are explored for blue OLEDs, designing efficient blue monochromatic active material for OLEDs is still a major challenge. Blue emitters are large band gap materials, which cause serious mismatches across neighboring layers when used as an active device component leading to high operational current and Joule heating. The color purity of blue light emitters does not meet the acceptable display technology standards. Moreover, monochromic blue light emitters have low stability and high roll-off efficiency.72,73 Because of the high band gap in these blue emitters, doping plays a valuable role, and efficiencies are increased in doped devices compared with undoped ones. Although Schiff bases with metal atoms such as Zn, Pt, Mn, and Fe display high thermal stabilities and better emission properties, the development of efficient blue emitters with optimal band gap is necessary to produce better blue OLEDs with high efficiency and low operational current. Azomethine-Zn complex 3 showed maximum luminance value, current efficiency, and power efficiency among the blue emitters reported for OLED application.
Green Emitters
Green is one of the primary colors used in full-color display technology. The popular Al complexes and their derivatives like Tris(8-hydroxyquinolinato)aluminium (Alq3) are metal-chelates commonly used as active EML and electron transporting systems for device applications.55,74,75 Some Schiff base complexes with different metal centers such as Ni, W, and Zn were proposed as green light-emitting materials with higher QY compared to Alq3.
Lepnev et al. fabricated an OLED with Zn complexes of tetradentate Schiff bases obtained from salicylaldehyde derivatives 7a-b and o-vanillin derivatives 8a-b as EMLs.76 (Fig. 5a) The device with architecture ITO|α-NPD (4,4’-bis[N-(1-naphthyl)-N-phenylamino]biphenyl)|7a-b or 8a-b|Ca|Al underwent reversible degradation owing to the charge trapping process. The release of a charge carrier with the retrieval of EL intensity and lifetime can be realized by heating the OLEDs, exposure to short UV light, switching off the voltage or changing to alternating voltage. The irreversible degradation of OLEDs under prolonged heating occurred owing to changes in the interface regions and also during aging at ambient conditions. The OLEDs did not degrade under low UV irradiation, and hence displayed promising prospects under daylight operation. The OLED working characteristics were dependent on the morphological properties of EMLs. The substrate temperature and decrease in evaporation velocity facilitated even surfaces for the thin film of the complexes, and therefore, led to the disappearance of the initial decrease in EL intensity and later jumps after ‘time pauses’. However, these OLEDs were not adequately brighter and their performances decreased with aging. The luminescence of these devices was ~50 cd m−2 and it can be further improved by stopping the progress in the degradation process.
Green emitting Schiff base complexes and their device architectures: (a) Zinc complexes with tetradentate Schiff bases (data from Ref. 76), (b) Tungsten Schiff bases with high QY and TADF (data from Ref. 77), (c) & (d) Nickel-Schiff base complexes (data from Refs. 78 and 79) and (e) Salicylaldehyde Schiff base derivatives (data from Ref. 80).
Chan et al. proposed a tungsten (W) complex with Schiff base as a thermally activated delayed fluorescence (TADF) emitter for a green OLED with high QY up to 84%.77 (Fig. 5b) The Schiff base ligand was synthesized by condensing substituted salicylaldehyde with 2,2-dimethylpropane1,3-diamine and addition of WO2Cl2 in the presence of triethylamine generating the complexes 9, 10a-b and 11a-d (11c: λab = 297, 407 nm; λem = 608 nm, 11d: λab = 304, 405 nm; λem =554 nm) with high thermal stability. The thin films of the complexes with 5 wt.% of mCP displayed a QY up to 84% and the fluorescence lifetime of 2.0 µs. These complexes were used as emitting material to fabricate devices with configuration ITO|PEDOT:PSS|PVK(Polymethylmethacrylate):OXD-7(1,3-bis[(4-tert-butylphenyl)-1,3,4 oxadiazolyl]phenylene):9 or 10a-b or 11a-d|TPBi|LiF|Al, which had a QY of 15.56% for complex 11d at 30 wt.% dopant with maximum luminance of 16,890 cd m−2.
Kara et al. synthesized a Schiff base ligand by condensing 3-aminopropan-1-ol with 3,5-dichlorosalicylaldehyde and a green-emitting nickel (Ni) complex by refluxing the ligand with nickel (II) acetate tetrahydrate 12 (Fig. 5c). The complex displayed ILCT and chelation enhanced fluorescence (CHEF), which can be used in solid-state lighting applications. Yet another green-emitting Ni complex was synthesized by Srinivas et al. by reacting 4-butylaniline with an ethanolic solution of 2-hydroxynaphthalene-1-carbaldehyde, and subsequent complexation was done by reacting the ligand with NiCl2.6H2O.79 (Fig. 5d) Complex 13 displayed a triclinic space group with a single molecule in the asymmetric unit. The complex with optical band gap 1.83 eV and CIE coordinates (0.27634, 0.5623) can be used in OLED devices. Mohan et al. synthesized five Schiff bases 14a-e which can be used as emitting material in fabricating an OLED.80 (Fig. 5e) Compounds 14b and 14d strongly emitted green light at 512 and 520 nm, respectively, in solid-state, with electrochemical band gaps in the range of 2.25-2.32 eV. The QY and fluorescence lifetime provided evidence for 14b as a potential candidate for the fabrication of green OLED.
Suresh et al. synthesized 2-(N-aryl)formiminopyrrolyl boron complexes 15 (λab = 383 nm; λem = 451 nm) and 16a-b (16a: λab = 428 nm; λem = 512 nm, 16b: λab = 419 nm; λem = 497 nm) by reacting triphenylborane with 2-(N-aryl)formiminopyrrole ligand in toluene and utilized to fabricate single layer doped and undoped devices.81 (Fig. 6a) Devices with architecture ITO|PEDOT:PSS |15/16a-b or 15/16a-b:OXD-7|Ca/Ba|Al were prepared to obtain a maximum luminance of ~1000 cd m−2 with EQE of 0.3 cd A−1. Increased wt.% of 1,3-bis[(4-tert-butylphenyl)-1,3,4-oxadiazolylphenylene (OXD-7) in the EML increased the EQE with a maximum of 0.67 cd/A for 16a due to the charge trapping property of OXD-7. Ca was a better electron transferring agent than Ba in the fabricated devices. Liu et al. in 2010 synthesized N-arylanilidoarylimine bidentate Schiff base ligands by reacting fluorobenzaldehyde with arylamine.82 (Fig. 6b) These ligands were reacted with n-BuLi to obtain lithium (Li) complex and then Li was replaced by B on reacting with BF3(OEt2) to form boron complexes 17a-c. Among three complexes 17c (λab = 438 nm; λem = 515 nm) showed the highest QY and hence was used as a dopant with the well-known host PVK. Four devices were prepared by varying 17c dopant concentration from 2-8 wt.%. Maximum efficiency was observed from the device with 8 wt.% with a maximum quantum efficiency of 2.92 cd A−1. Additionally, a higher concentration of the dopant led to a decrease in efficiency.
Later in 2015 Suresh et al. synthesized various boron complexes 18-24 with Schiff bases obtained from 2-formylpyrrole and aromatic di- and tri-amines.65 (Fig. 7) Though the precursor Schiff bases were non-emissive, the boron complexes were green to yellow emissive, where the emission color was based on the chain length of π-conjugation. Longer π-conjugation length led to the emission of a green color, whereas a shorter conjugation length led to blue emission. Different single-layered devices were fabricated by spin coating or sublimation with configuration ITO|PEDOT:PSS|18-24|Ba/Ca|Al with a maximum luminescence of 958 cd m−2 and 0.084% of EQE with 19 (λab = 428 nm; λem = 512 nm). Usage of Ba was beneficial in only devices incorporating 18 (λab = 400 nm; λem = 468 nm) and 20 (λab = 419 nm; λem = 496 nm). The devices obtained through sublimation showed better performance than the spin-coated ones except in the case of 19. The device performance was made better by inserting TPD which led to current reduction and better hole-electron balance showing 2000 cd m−2 of maximum luminescence and 0.145% of EQE. Most of the sublimed devices with Ba and TPD displayed higher EQE and maximum luminescence.
2-Formylpyrrole-based boron complexes as green-yellow emitters and their device architecture (data from Ref. 65).
In the fabrication processes, solubility and stability in different solvents are considerably important aspects. The complexes with heteroatoms and alkyl side chains displayed better solubility, which can be used in solution-processed systems. Substituted salicylaldehydes were mainly used to prepare the ligands in these green-light emitters. Longer π-conjugation length can result in green emission. The intensity of emission and color was tuned based on the conjugated chain length. PVK, OXD-7, and a blend of these two compounds were utilized as a host material in devices with green-emitting Schiff base complexes, which enhanced the device performance due to charge trapping capabilities and facilitated high charge mobility. The tungsten-based complexes displayed better performance among all the Schiff base chelates reported as green-emitting materials for OLED application.
Red Emitters
Deep red OLEDs find application in different areas including flexible room lamps, phototherapy, laptop sources, foldable smartphones screens, biomedical and telecommunication, or security and biological sensors due to their possible miniaturization, simple integration, and cost-effective features.83,84,85 The development of red emitters is the toughest task according to energy law because of energy loss and low IQE attributed to triplet-triplet annihilation and self-quenching.63,86
1,8-Naphthalimide serves as a hole-blocking and electron-conducting material. Gan et al. synthesized two series of Schiff bases 25a-e and 26a-b through condensation reaction between four hydrazino-naphthalimides with various aldehydes.87 (Fig. 8) These azomethine derivatives exhibited intramolecular charge transfer between the electron-donating amine group and the electron-deficient naphthalimide unit. The extended amino conjugation at the imide nitrogen and the presence of electron-donating units attached to the naphthalimide framework resulted in emission showing a redshift of these Schiff base films. The OLED with ITO|CuPc (12 nm)| NPB (30 nm)|25d (45 nm)|sodium stearate (2 nm)|Al (100 nm) as device architecture produced a maximum luminance of 15.5 cd/m2 and current density of 2.9 mA/cm2, at an applied voltage of 22 V. Phthalocyanine copper (CuPc) was used as a hole transporting layer to balance the electron and hole injection. The device with undoped 25d (λab = 464 nm; λem= 661 nm) as EML displayed relatively poor performance.
An electron-deficient red-emitting 1,8-naphthalimide-based Schiff base as an emitter in an undoped OLED (data from Ref. 87).
Che et al. synthesized various Pt-Schiff base complexes 27a-b, 28, 29a-d, 30a-c, 31a-b, 32, 33, 34a-b by reacting ligands with K2PtCl4 to fabricate phosphorescent yellow-red organic OLEDs (Fig. 9). The thermally stable molecules with intermolecular π-π stacking interactions were organized in a head-to-tail manner.57 The devices with ITO|NPB|CBP|BCP(bathocuproine)|Alq3|LiF|Al architecture incorporated the metal complexes as a guest in the host CBP and the device efficiency increased with low dopant concentration. The best efficiency of up to 31 cd A−1 and a device lifetime up to 77,000 h at 500 cd m−2 was obtained.
Red emitting phosphorescent Pt(II) complexes with device architecture (data from Ref. 57).
Blondel et al. synthesized two Pt-based salophen-type complexes 35 and 36 by reacting 3,5-di-tert-butylsalicylaldehyde or salicylaldehyde with 1,2-phenylenediamine and subsequent complexation reaction with K2PtCl4.86 (Fig. 10) These complexes, which exhibited high thermal stability were utilized as dopants with Alq3 as the HOMO levels of both 35 and 36 were higher, and the LUMO levels were lower than Alq3. The tertbutyl substituents limited the stacking of complex 35 due to steric hindrance and promoted efficient charge and electron transport from the host Alq3 matrix to the Pt(II) complex. Thus the enhancement in quantum efficiency and device performance was mainly due to the tert-butyl substituents that reduced the aggregation-induced phosphorescence quenching in the Alq3/Pt(II) complex-based layer. The Alq3/Pt(II) complex monolayer displayed negative differential resistance, and this conductance modulation of the active layer was due to the electrically active defects formed within the Alq3 layer. The defects were induced by Pt(II) complexes that participate in the charge balance. The device I was fabricated with Alq3 as emitting material. However, devices II and III with 5 % of 35 and 36 doped with Alq3, respectively, showed EL in a deep-red region with (0.690, 0.309) and (0.655, 0.343) CIE coordinates and turn-on voltages of 5 V and 6 V, respectively. The electronic states of Pt(II) complexes affected the trapping ability and charge mobility. The highest exciton quenching was observed within the Alq3/36 layer leading to lower device performance of device III than device II. Devices IV, V, and VI were monolayer devices without NPD, wherein device VI showed both the emission of Alq3 and 36. Devices V and VI exhibited better performance than device IV.
Sterically hindered red-emitting Pt(II) complex without aggregation-induced quenching (data from Ref. 86).
It is observed from the above investigations that both doped and undoped Schiff bases are used as EMLs in devices. Pt(II) Schiff base complexes are used as dopant materials in phosphorescent OLEDs for their stability and emission in the range from yellow to red.52,57,58,88,89 Platinum complexes showed higher luminance value and current efficiency with better performance for OLED application.
White Emitters
The white organic light-emitting diodes (WOLED) were fabricated by blending different colored fluorescent dyes such as red, green, and blue in poly(N-vinylcarbazole). These diodes are broadly used in full-color screens, LCD backlights and ambient lighting. Because of high performance, thermal stability, simple tunability of color, and their cost-effective nature, Zn complexes are extensively used in fabricating WOLEDs. These possess lower molecular weight and can sublimate in vacuum easily. Five Zn metal-chelate complexes 37a-c (37a: λab = 260, 356 nm; λem = 464 nm, 37b: λab = 253, 360 nm; λem = 468 nm, 37c: λab = 255, 356 nm; λem = 430 nm) and 38a-b (38a: λab = 265, 360 nm; λem = 444 nm, 38b: λab = 268, 357 nm; λem = 468nm) based on tetradentate Schiff bases were synthesized by Dumur et al. as dopants for white light-emitting material as well as pure emitter to produce multilayer p-i OLED.90(Fig. 11) The Schiff base complexes were prepared by reacting substituted diamines with salicylaldehyde and zinc acetate. Devices with configuration ITO|MeOTPD:F4TCNQ|MeOTPD|TCTA(4,4’,4”-Tri(N-carbazolyl)triphenylamine):37a-c and 38a-b or 37a-c and 38a-b|BCP|TPBi|LiF|Al were fabricated. Complex 37a dimerized to produce a yellow OLED. The steric hindrance of the long diamine unit of 38a resulted in the deep-blue emission of OLEDs. WOLEDs were fabricated by doping these complexes with 4,4′,4″-tris(N-carbazolyl)triphenylamine to achieve high-quality white-light emission with CIE coordinates (0.33, 0.34). Among all devices, TCTA:37b showed maximum brightness of 815 cdm−2 (8.6 V) having CIE coordinates (0.26, 0.34) with 10% of doping.
Zinc-Schiff base complexes for white organic light-emitting devices (data from Ref. 90).
Summary
The review is mainly focused on the synthesis of various Schiff bases and their complexes with different metals such as Zn, Pt, Mn, Fe and W, which can be used as doped or undoped layers of OLEDs. The different device architectures using these luminescent materials as potential candidates for EMLs and also as dopants for mCP, PVK, OXD-7, Alq3, and TCTA are illustrated and the respective device performances are summarized in Table I. Devices emitting different colors such as blue, green, red, and white were prepared from Schiff bases and their complexes by tuning the wavelength of emissive material through incorporation of various substituent groups and metal atoms to make them highly luminescent and thermally stable.
Future Prospects
Despite steady progress achieved in the fabrication of OLEDs, designing efficient monochromatic blue luminophores as active materials remains a challenge because these blue light-emitting materials possess a wide electronic band gap that leads to the mismatch in energy levels with the adjacent layer in the device. High operational voltage is required to overcome this energy mismatch, causing Joule heating leading to material degradation on prolonged exposure, reduced device lifetime, poor stability, and high roll-off efficiency.72,91 Moreover, the color purity of such devices does not meet the standard values of display technology. Production of white light emission with such blue emitters also becomes a challenge72. Therefore, the development of a luminophore possessing high solid-state QY is necessary to produce highly effective blue OLEDs.68,92,93,94,95,96,97 To achieve improved deep blue narrow luminescence characteristics, the dopant concentration can be altered and lowers the excimer emission of the host matrix, which in turn decreases the y coordinate on the CIE plot. Additionally, the thickness of the active layers can be reduced to improve power and current efficiencies and to reduce the current density parameters. The development of red-emitting devices is quite difficult because of the lower band gap.98,99,100
The operational stability of OLEDs is yet another crucial issue for their effective and extensive application for practical purposes, especially in displays and for lighting. Therefore, further research to enhance this parameter is also very vital. Though immense progress was observed in OLED material research and devices, the clear mechanism behind the intrinsic OLED degradation is unknown.101 For practical application, both operational stability and the mechanism behind the degradation are important. We hope that this review illustrating the different types of Schiff bases and their complexes that have been explored to develop OLEDs with improved properties highlight the need for further improvements in this direction for achieving longer-lived devices with superior features.
Abbreviations
- Al:
-
Aluminium
- Alq3 :
-
Tris-(8-hydroxyquinoline)aluminum
- α-NPD:
-
(4,4’-Bis[N-(1-naphthyl)-N-phenylamino]biphenyl
- B:
-
Boron
- BCP:
-
Bathocuproine
- Ba:
-
Barium
- Ca:
-
Calcium
- CAB:
-
Cellulose acetate butyrate
- CBP:
-
4,4’-N,N’-Dicarbazolylbiphenyl
- CE:
-
Current efficiency
- CHEF:
-
Chelation enhanced fluorescence
- CIE:
-
Commission international de l’éclairge
- CuPc:
-
Phthalocyanine copper
- EL:
-
Electroluminescence
- EML:
-
Emissive layer
- EQE:
-
External quantum efficiency
- HOMO:
-
Highest occupied molecular orbital
- ILCT:
-
Intra-ligand charge transfer
- ISC:
-
Intersystem crossing
- ITO:
-
Indium tin oxide
- LCD:
-
Liquid crystal display
- K2PtCl4 :
-
Potassium tetrachloroplatinate
- Li:
-
Lithium
- LiF:
-
Lithium fluoride
- LLCT:
-
Ligand-to-ligand charge transfer
- LUMO:
-
Lowest unoccupied molecular orbital
- mCP:
-
1,3-Bis-(N-carbazolyl)benzene
- MLCT:
-
Metal-to-ligand charge transfer
- Mn:
-
Manganese
- Ni:
-
Nickel
- NPB:
-
N,N’-Bis(1-naphthyl)-N,N’-diphenyl-1,1’-biphenyl- 4,4’-diamine
- OLED:
-
Organic light-emitting diode
- OXD-7:
-
1,3-Bis[(4-tert-butylphenyl)-1,3,4-oxadiazolyl]phenylene
- PBD:
-
(2-4-Biphenylyl)-5-phenyl-oxadiazole
- PE:
-
Power efficiency
- PEDOT:
-
Poly(3,4-ethylenedioxythiophene)
- PL:
-
Photoluminescence
- PMMA:
-
Polymethylmethacrylate
- PSS:
-
Polystyrene sulfonate
- Pt:
-
Platinum
- PVK:
-
Polyvinylcarbazole
- QY:
-
Quantum yield
- TADF:
-
Thermally activated delayed fluorescence
- TCTA:
-
4,4’,4”-Tri(N-carbazolyl)triphenylamine
- TPBi:
-
1,3,5-Tris(1-phenyl-1H-benzo[d]imidazol-2-yl)benzene
- TPD:
-
N,N’-Bis(3-methylphenyl)-N,N’-diphenyl-1,1’-biphenyl-4,4’-diamine
- W:
-
Tungsten
- WOLED:
-
White organic light-emitting diode
- Zn:
-
Zinc
References
N. Zhang, F. Huang, S. Zhao, X. Lv, Y. Zhou, S. Xiang, S. Xu, Y. Li, G. Chen, C. Tao, Y. Nie, J. Chen, and X. Fan, Photo-rechargeable fabrics as sustainable and robust power sources for wearable bioelectronics. Matter 2, 1260 (2020).
Z. Zhou, K. Chen, X. Li, S. Zhang, Y. Wu, Y. Zhou, K. Meng, C. Sun, Q. He, W. Fan, E. Fan, Z. Lin, X. Tan, W. Deng, J. Yang, and J. Chen, Sign-to-speech translation using machine-learning-assisted stretchable sensor arrays. Nat. Electron. 3, 571 (2020).
J. Chen, Y. Huang, N. Zhang, H. Zou, R. Liu, C. Tao, X. Fan, and Z.L. Wang, Micro-cable structured textile for simultaneously harvesting solar and mechanical energy. Nat. Energy 1, 1 (2016).
K. Meng, S. Zhao, Y. Zhou, Y. Wu, S. Zhang, Q. He, X. Wang, Z. Zhou, W. Fan, X. Tan, J. Yang, and J. Chen, A wireless textile-based sensor system for self-powered personalized health care. Matter 2, 896 (2020).
G. Chen, Y. Fang, X. Zhao, T. Tat, and J. Chen, Textiles for learning tactile interactions. Nat. Electron. 4, 175 (2021).
Z. Zhou, S. Padgett, Z. Cai, G. Conta, Y. Wu, Q. He, S. Zhang, C. Sun, J. Liu, E. Fan, K. Meng, Z. Lin, C. Uy, J. Yang, and J. Chen, Single-layered ultra-soft washable smart textiles for all-around ballistocardiograph, respiration, and posture monitoring during sleep. Biosens. Bioelectron. 155, 112064 (2020).
P. Fassl, V. Lami, F.J. Berger, L.M. Falk, J. Zaumseil, B.S. Richards, I.A. Howard, Y. Vaynzof, and U.W. Paetzold, Revealing the internal luminescence quantum efficiency of perovskite films via accurate quantification of photon recycling. Matter 4, 1391 (2021).
T. Tat, A. Libanori, C. Au, A. Yau, and J. Chen, Advances in triboelectric nanogenerators for biomedical sensing. Biosens. Bioelectron. 171, 112714 (2021).
B. Zhang, F. Chun, G. Chen, T. Yang, A. Libanori, K. Chen, G. Conta, D. Xiong, W. Yang, and J. Chen, Water-evaporation-induced intermolecular force for nano-wrinkled polymeric membrane. Cell Rep. Phys. Sci. 2, 100441 (2021).
Q. Xu, Y. Fang, Q. Jing, N. Hu, K. Lin, Y. Pan, L. Xu, H. Gao, M. Yuan, L. Chu, Y. Ma, Y. Xie, J. Chen, and L. Wang, A portable triboelectric spirometer for wireless pulmonary function monitoring. Biosens. Bioelectron. 187, 113329 (2021).
M.C. Roco, M.C. Hersam, C.A. Mirkin, Nanotechnology research directions for societal needs in 2020. (2011), pp. 261–303
G. Chen, Y. Li, M. Bick, and J. Chen, Smart textiles for electricity generation. Chem. Rev. 120, 3668 (2020).
A. Taparugssanagorn, S. Siwamogsatham, and C. Pomalaza-Ráez, A MISO UCA beamforming dimmable LED system forindoor positioning. Sensors 14, 2362 (2014).
W. Yang, Z. Liu, J. Chen, L. Huang, L. Zhang, H. Pan, B. Wu, and Y. Lin, A High-performance white-light-emitting-diodes based on nano-single crystal divanadates quantum dots. Sci. Rep. 5, 1 (2015).
U. Guler, J.C. Ndukaife, G.V. Naik, A.G.A. Nnanna, A.V. Kildishev, V.M. Shalaev, and A. Boltasseva, Local heating with lithographically fabricated plasmonic titanium nitride nanoparticles. Nano Lett. 13, 6078 (2013).
H.J. Bowlden, Radiative transitions in semiconductors. Phys. Rev. 106, 427 (1957).
W. Zhao, Z. He, J.W.Y. Lam, Q. Peng, H. Ma, Z. Shuai, G. Bai, J. Hao, and B.Z. Tang, Rational molecular design for achieving persistent and efficient pure organic room-temperature phosphorescence. Chem 1, 592 (2016).
Z. Ren, and S. Yan, Polysiloxanes for optoelectronic applications. Prog. Mater. Sci. 83, 383 (2016).
J. Jiang, A. Trewin, D.J. Adams, and A.I. Cooper, Band gap engineering in fluorescent conjugated microporous polymers. Chem Sci. 2, 1777 (2011).
Z. Dechun, Chemical and photophysical properties of materials for OLEDs. (Woodhead Publishing Limited, 2013).
D.A. Xavier, and N. Srividhya, Synthesis and study of Schiff base ligands. IOSR J. Appl. Chem. 7, 6 (2014).
C.W. Tang, and S.A. Vanslyke, Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913 (1987).
C. Adachi, S. Tokito, T. Tsutsui, and S. Saito, Electroluminescence in organic films with three-layer structure. Jpn. J. Appl. Phys. 27, L269 (1988).
B.A. Endo, M. Ogasawara, A. Takahashi, D. Yokoyama, Y. Kato, and C. Adachi, Thermally activated delayed fluorescence from Sn4+ -porphyrin complexes and their application to organic light-emitting diodes- A novel mechanism for electroluminescence. Adv. Mater. 21, 4802 (2009).
W. Wu, J. Sun, S. Ji, W. Wu, J. Zhao, and H. Guo, Tuning the emissive triplet excited states of platinum(II) Schiff base complexes with pyrene, and application for luminescent oxygen sensing and triplet-triplet-annihilation based upconversions. Dalt. Trans. 40, 11550 (2011).
M.A. Baldo, D.F. O’Brien, Y. You, A. Shoustikov, S. Sibley, M.E. Thompson, and S.R. Forrest, Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151 (1998).
S.V. Eliseeva, and J.C.G. Bünzli, Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 39, 189 (2010).
K. Binnemans, Lanthanide-based luminescent hybrid materials. Chem. Rev. 109, 4283 (2009).
A. De Bettencourt-Dias, Lanthanide-based emitting materials in light-emitting diodes. J. Chem. Soc. Dalt. Trans. 22, 2229 (2007).
A. Buyruk, M.E. Cinar, M.S. Eroglu, and T. Ozturk, Polymerization of thienothiophenes and dithienothiophenes via click-reaction for electronic applications. ChemistrySelect 1, 3028 (2016).
S. Hadsadee, R. Rattanawan, R. Tarsang, N. Kungwan, and S. Jungsuttiwong, Push-pull N-annulated perylene-based sensitizers for dye-sensitized solar cells: Theoretical property tuning by DFT/TDDFT. ChemistrySelect 2, 9829 (2017).
Z. Hu, H. Zhang, Y. Chen, Q. Wang, M.R.J. Elsegood, S.J. Teat, X. Feng, M.M. Islam, F. Wu, and B.Z. Tang, Tetraphenylethylene-based color-tunable AIE-ESIPT chromophores. Dyes. Pigm. 175, 108175 (2020).
D. Sek, M. Siwy, K. Bijak, M. Grucela-Zajac, G. Malecki, K. Smolarek, L. Bujak, S. Mackowski, and E. Schab-Balcerzak, Comparative studies of structural, thermal, optical, and electrochemical properties of azines with different end groups with their azomethine analogues toward application in optoelectronics. J. Phys. Chem. A 117, 10320 (2013).
J. Shi, and C.W. Tang, A Anthracene derivatives for stable blue-emitting organic electroluminescence devices. Appl. Phys. Lett. 80, 3200 (2002).
A.K. Satapathy, S.K. Behera, A. Yadav, L.N. Mahour, C.V. Yelamaggad, K.L. Sandhya, and B. Sahoo, Tuning the fluorescence behavior of liquid crystal molecules containing Schiff-base: Effect of solvent polarity. J. Lumin. 210, 371 (2019).
A.K. Satapathy, S.K. Behera, R. Kumar, K.L. Sandhya, C.V. Yelamaggad, and B. Sahoo, Excited state intramolecular proton transfer emission in bent core liquid crystals. J. Photochem. Photobiol. A Chem. 358, 186 (2018).
K. Muzammil, P. Trivedi, and D.B. Khetani, Synthesis and characterization of Schiff base m-nitroaniline and their complexes. Res. J. Chem. Sci. Res. J. Chem. Sci. 5, 2231 (2015).
R.S. Varma, R. Dahiya, and S. Kumar, Clay catalyzed synthesis of imines and enamines under solvent free conditions using microwave irradiation. Tetrahedron Lett. 38, 2039 (1997).
J. Schmeyers, F. Toda, J. Boy, and G. Kaupp, Quantitative solid-solid synthesis of azomethines. J. Chem. Soc. Perkin Trans. 2, 989 (1998).
A. Vass, J. Dudas, and R.S. Varma, Solvent-free synthesis of N-Sulfonylimines using microwave irradiation. Tetrahydron Lett. 40, 4951 (1999).
K. Tanaka, and R. Shiraishi, Clean and efficient condensation reactions of aldehydes and amines in a water suspension medium. Green Chem. 2, 272 (2000).
C. Andrade, S. Takada, L. Alve, J. Rodrigues, P. Suarez, R. Brandao, and V. Soares, Molecular sieves in ionic liquids as an efficient and recyclable medium for the synthesis of imines. Synth. Lett. 12, 2135 (2005).
M.Á. Vázquez, M. Landa, R. Miranda, and J. Tamariz, Infrared irradiation: Effective promoter in the formation of N-Benzylideneanilines in the absence of solvent. Synth. Commun. 34, 2705 (2009).
M. Gopalakrishnan, P. Sureshkumar, V. Kanagarajan, J. Thanusu, and R. Govindaraju, Silica gel supported sodium hydrogen sulfate as an efficient and reusable heterogeneous catalyst for the synthesis of imines in solvent-free conditions under microwave irradiation. J. Chem. Res. 2005, 299 (2005).
M. Gopalakrishnan, P. Sureshkumar, V. Kanagarajan, and J. Thanusu, New environmentally-friendly solvent-free synthesis of imines using calcium oxide under microwave irradiation. Res. Chem. Intermed. 33, 541 (2007).
C.M. Da Silva, D.L. Da Silva, L.V. Modolo, R.B. Alves, M.A. De Resende, C.V.B. Martins, and Â. De Fátima, Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2, 1 (2011).
A.M. Abu-Dief, and I.M.A. Mohamed, A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 4, 119 (2015).
A.F. Rausch, H. Homeier, P.I. Djurovich, M.E. Thompson, and H. Yersin, Spin-orbit coupling routes and OLED performance: Studies of blue-light emitting Ir(III) and Pt(II) complexes. Org. Light Emit. Mater. Devices XI 6655, 66550 (2007).
P.K. Chow, C. Ma, W.P. To, G.S.M. Tong, S.L. Lai, S.C.F. Kui, W.M. Kwok, and C.M. Che, Strongly phosphorescent palladium(II) complexes of tetradentate ligands with mixed oxygen, carbon, and nitrogen donor atoms: Photophysics, photochemistry, and applications. Angew. Chemie - Int. Ed. 52, 11775 (2013).
A.F. Rausch, L. Murphy, J.A.G. Williams, and H. Yersin, Improving the performance of Pt(II) complexes for blue light emission by enhancing the molecular rigidity. Inorg. Chem. 51, 312 (2012).
P.A. Vigato, and S. Tamburini, The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 248, 1717 (2004).
J. Zhang, G. Dai, F. Wu, D. Li, D. Gao, H. Jin, S. Chen, X. Zhu, C. Huang, and D. Han, Efficient and tunable phosphorescence of new platinum(II) complexes based on the donor-π-acceptor Schiff bases. J. Photochem. Photobiol. A Chem. 316, 12 (2016).
C. Du, S. Ye, Y. Liu, Y. Guo, T. Wu, H. Liu, J. Zheng, C. Cheng, M. Zhu, and G. Yu, Fused-seven-ring anthracene derivative with two sulfur bridges for high performance red organic light-emitting diodes. Chem. Commun. 46, 8573 (2010).
H. Yersin, Highly efficient OLEDs with phosphorescent materials, (Wiley-VCH, 2008).
A.F. Rausch, M.E. Thompson, and H. Yersin, Blue light emitting Ir(III) compounds for OLEDs-new insights into ancillary ligand effects on the emitting triplet state. J. Phys. Chem. A 113, 5927 (2009).
J.S. Wilson, N. Chawdhury, M.R.A. Al-mandhary, M. Younus, M.S. Khan, P.R. Raithby, A. Ko, and R.H. Friend, The energy gap law for triplet states in Pt-containing conjugated polymers and monomers. J. Am. Chem. Soc 123, 9412 (2001).
C.M. Che, C. Kwok, S.W. Lai, A.F. Rausch, W.J. Finkenzeller, N. Zhu, and H. Yersin, Photophysical properties and OLED applications of phosphorescent platinum(II) Schiff base complexes. Chem. - A Eur. J. 16, 233 (2010).
L. Zhou, C.L. Kwong, C.C. Kwok, G. Cheng, H. Zhang, and C.M. Che, Efficient red electroluminescent devices with sterically hindered phosphorescent platinum(II) Schiff base complexes and iridium complex codopant. Chem. - An Asian J. 9, 2984 (2014).
C.M. Che, S.C. Chan, H.F. Xiang, M.C.W. Chan, Y. Liu, and Y. Wang, Tetradentate Schiff base platinum(II) complexes as new class of phosphorescent materials for high-efficiency and white-light electroluminescent devices. Chem. Commun. 4, 1484 (2004).
D.F. O’Brien, M.A. Baldo, M.E. Thompson, and S.R. Forrest, Improved energy transfer in electrophosphorescent devices. Appl. Phys. Lett. 74, 442 (1999).
T. Sano, Y. Nishio, Y. Hamada, H. Takahashi, T. Usuki, and K. Shibata, Design of conjugated molecular materials for optoelectronics. J. Mater. Chem. 10, 157 (2000).
Y. Hamada, T. Sano, H. Fujii, Y. Nishio, H. Takahashi, and K. Shibata, Organic light-emitting diodes using 3- or 5-Hydroxyflavone-metal complexes. Appl. Phys. Lett. 71, 3338 (1997).
O. Lavastre, I. Illitchev, G. Jegou, and P.H. Dixneuf, Discovery of new fluorescent materials from fast synthesis and screening of conjugated polymers. J. Am. Chem. Soc. 124, 5278 (2002).
A.C. Leung, J.H. Chong, B.O. Patrick, and M.J. Maclachlan, Poly(salphenyleneethynylene)s: A new class of soluble, conjugated, metal-containing polymers. Macromolecules 36, 5051 (2003).
D. Suresh, C.S.B. Gomes, P.S. Lopes, C.A. Figueira, B. Ferreira, P.T. Gomes, R.E.D. Paolo, A.L. MaçAnita, M.T. Duarte, A. Charas, J. Morgado, D. Vila-Viçosa, and M.J. Calhorda, Luminescent di- and trinuclear boron complexes based on aromatic iminopyrrolyl spacer ligands: Synthesis, characterization, and application in OLEDs. Chem. - A Eur. J. 21, 9133 (2015).
M. Mohan, R. John, S.M. Nagarajan, and D.R. Trivedi, Design, Synthesis and characterization of N-substituted heteroaromatics: DFT-studies and organic light emitting device application. ChemistrySelect 5, 5903 (2020).
J. Zhang, X. Zhu, A. Zhong, W. Jia, F. Wu, D. Li, H. Tong, C. Wu, W. Tang, P. Zhang, L. Wang, and D. Han, New platinum(II) one-armed schiff base complexes for blue and orange PHOLEDs applications. Org. Electron. 42, 153 (2017).
A.N. Gusev, M.A. Kiskin, E.V. Braga, M. Chapran, G. Wiosna-Salyga, G.V. Baryshnikov, V.A. Minaeva, B.F. Minaev, K. Ivaniuk, P. Stakhira, H. Ågren, and W. Linert, Novel zinc complex with an ethylenediamine Schiff base for high-luminance blue fluorescent OLED applications. J. Phys. Chem. C 123, 11850 (2019).
J.S. Kang, J.G. Kang, Y. Sohn, and K.T. Leung, Blue-light-emitting photostable hybrid films for high-efficiency large-area light converter photonic applications. ACS Appl. Mater. Interfaces 10, 44768 (2018).
N.K. Gondia, J. Priya, and S.K. Sharma, Spectroscopic investigation and luminescent properties of Schiff base metal complex for OLED. AIP Conf. Proc. 1953, 1 (2018).
A. Donmez, M.B. Coban, and H. Kara, Cyan-blue luminescence and antiferromagnetic coupling of CN-bridged tetranuclear complex based on manganese(III) Schiff base and hexacyanoferrate(III). J. Clust. Sci. 29, 951 (2018).
S. Kim, H.J. Bae, S. Park, W. Kim, J. Kim, J.S. Kim, Y. Jung, S. Sul, S.G. Ihn, C. Noh, S. Kim, and Y. You, Degradation of blue-phosphorescent organic light-emitting devices involves exciton-induced generation of polaron pair within emitting layers. Nat. Commun. 9, 1 (2018).
C.S. Oh, J.M. Choi, and J.Y. Lee, Chemical bond stabilization and exciton management by cn modified host material for improved efficiency and lifetime in blue phosphorescent organic light-emitting diodes. Adv. Opt. Mater. 4, 1281 (2016).
Y. Qiu, Y. Shao, D. Zhang, and X. Hong, Preparation and characterization of high efficient blue-light emitting materials with a secondary ligand for organic electroluminescence. Japanese J. Appl. Phys. Part 1 39, 1151 (2000).
H. Kunkely, and A. Vogler, Optical properties of boron, gallium and gold complexes with salen ligands. emission from intraligand excited states under ambient conditions. Inorganica Chim. Acta. 321, 171 (2001).
L. Lepnev, A. Vaschenk, O.A. Vitukhnovsky, S. Eliseeva, O. Kotova, and N. Kuzmina, OLEDs based on some mixed-ligand terbium carboxylates and zinc complexes with tetradentate Schiff bases: Mechanisms of electroluminescence degradation. Synth. Met. 159, 625 (2009).
K.T. Chan, T.L. Lam, D. Yu, L. Du, D.L. Phillips, C.L. Kwong, G.S.M. Tong, G. Cheng, and C.M. Che, Strongly luminescent tungsten emitters with emission quantum yields of up to 84 %: TADF and high-efficiency molecular tungsten OLEDs. Angew. Chemie - Int. Ed. 58, 14896 (2019).
D.A. Kara, A. Donmez, H. Kara, and M. Burak Coban, Structural and spectroscopic characterization of a new luminescent Ni(II) Complex: Bis{2,4-dichloro-6-[(2–2ydroxypropyl)iminomethyl]phenolato-Κ3O, N, O′}nickel(II) Acta Crystallogr. Sect. C Struct. Chem. 74, 901 (2018).
M. Srinivas, T.O. Shrungesh Kumar, K.M. Mahadevan, S. Naveen, G.R. Vijayakumar, H. Nagabhushana, M.N. Kumara, and N.K. Lokanath, Synthesis Crystal structure and photoluminescence study of green light emitting Bis(1[(4-butylphenyl)imino] methyl naphthalen-2-ol) Ni(II) complex. J. Sci. Adv. Mater. Dev. 1, 324 (2016).
M. Mohan, S. Pangannaya, M.N. Satyanarayan, and D.R. Trivedi, Photophysical and electrochemical properties of organic molecules: Solvatochromic effect and DFT studies. Opt. Mater. 77, 211 (2018).
D. Suresh, C.S.B. Gomes, P.T. Gomes, R.E.D. Paolo, A.L. MaçAnita, M.J. Calhorda, A. Charas, J. Morgado, and M. Teresa Duarte, Syntheses and photophysical properties of new iminopyrrolyl boron complexes and their application in efficient single-layer non-doped OLEDs prepared by spin coating. Dalt. Trans. 41, 8502 (2012).
X. Liu, Y. Ren, H. Xia, X. Fan, and Y. Mu, Synthesis, structures, photoluminescent and electroluminescent properties of boron complexes with anilido-imine ligands. Inorganica Chim. Acta 363, 1441 (2010).
A.K. Bansal, S. Hou, O. Kulyk, E.M. Bowman, and I.D.W. Samuel, Wearable organic optoelectronic sensors for medicine. Adv. Mater. 27, 7638 (2015).
A. Steude, M. Jahnel, M. Thomschke, M. Schober, and M.C. Gather, Controlling the behavior of single live cells with high density arrays of microscopic OLEDs. Adv. Mater. 27, 7657 (2015).
J. Shinar, R. Shinar, Organic light-emitting devices (OLEDs) and OLED-based chemical and biological sensors: An overview. J. Phys. D. Appl. Phys. 41, 133001 (2008)
B. Blondel, F. Delarue, M. Lopes, S. Ladeira-Mallet, F. Alary, C. Renaud, and I. Sasaki, Investigation of a sterically hindered Pt(II) complex to avoid aggregation-induced quenching: Applications in deep red electroluminescent and electrical switching devices. Synth. Met. 227, 106 (2017).
J. Gan, Q. Liang, X. Yuan, K. Chen, and H. Tian, 1,8-Naphthalimides for non-doping OLEDs: The Tunable emission color from blue. Green to Red J Photochem Photobiol A Chem 162, 399 (2004).
C. Fan, and C. Yang, Yellow/orange emissive heavy-metal complexes as phosphors in monochromatic and white organic light-emitting devices. Chem. Soc. Rev. 43, 6439 (2014).
J. Zhang, F. Zhao, X. Zhu, W.K. Wong, D. Ma, and W.Y. Wong, New phosphorescent platinum(II) Schiff base complexes for PHOLED applications. J. Mater. Chem. 22, 16448 (2012).
F. Dumur, L. Beouch, M.A. Tehfe, E. Contal, M. Lepeltier, G. Wantz, B. Graff, F. Goubard, C.R. Mayer, J. Lalevée, and D. Gigmes, Low-cost zinc complexes for white organic light-emitting devices. Thin Solid Films 564, 351 (2014).
C. Murawski, K. Leo, and M.C. Gather, Efficiency roll-off in organic light-emitting diodes. Adv. Mater. 25, 6801 (2013).
S.R. Forrest, P.E. Burrows, Z. Shen, G. Gu, V. Bulovic, and M.E. Thompson, The stacked OLED (SOLED): A new type of organic device for achieving high-resolution full-color displays. Synth. Met. 91, 9 (1997).
J.S. Cho, K. Uchida, N. Yoshioka, and K. Yamamoto, Electrical and magnetic properties of electro-oxidative polymerized poly(tris(thienylphenyl)amine). Sci. Technol. Adv. Mater. 5, 697 (2004).
H. Lee, B. Kim, S. Kim, J. Kim, J. Lee, H. Shin, J.H. Lee, and J. Park, Synthesis and electroluminescence properties of highly efficient dual core chromophores with side groups for blue emission. J. Mater. Chem. C 2, 4737 (2014).
D. Zhang, M. Cai, Z. Bin, Y. Zhang, D. Zhang, and L. Duan, Highly efficient blue thermally activated delayed fluorescent OLEDs with record-low driving voltages utilizing high triplet energy hosts with small singlet-triplet splittings. Chem. Sci. 7, 3355 (2016).
S.K. Pathak, M. Gupta, S.K. Pal, and A.S. Achalkumar, Hexacatenars exhibiting π-π driven supergelation, aggregation induced blue light emission and thermochromism. ChemistrySelect 1, 5107 (2016).
L. Jones, C.M. Pask, A. Kazlauciunas, M. Gulcur, and L. Lin, Synthesis and characterisation of fused-heterocyclic molecular rods: A combined experimental and theoretical study on diethynyl-dithienothiophenyl derivatives. ChemistrySelect 2, 5958 (2017).
J.V. Caspar, E.M. Kober, B.P. Sullivan, and T.J. Meyer, Application of energy gap law to decay of charge-transfer excited states J. Am. Chem. Soc. 104, 630 (1982).
S.D. Cummings, and R. Eisenberg, Tuning the excited-state properties of platnum(II) diimine dithiolate complexes. J. Am. Chem. Soc. 118, 1949 (1996).
C.H. Fan, P. Sun, T.H. Su, and C.H. Cheng, Host and dopant materials for idealized deep-red organic electrophosphorescence devices. Adv. Mater. 23, 2981 (2011).
H. Aziz, and Z.D. Popovic, Degradation phenomena in small-molecule organic light-emitting devices. Chem. Mater. 16, 4522 (2004).
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kagatikar, S., Sunil, D. Schiff Bases and Their Complexes in Organic Light Emitting Diode Application. J. Electron. Mater. 50, 6708–6723 (2021). https://doi.org/10.1007/s11664-021-09197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09197-9