Abstract
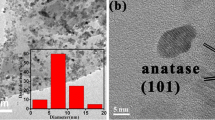
TiO2–reduced graphene oxide (RGO) nanocomposites have been prepared by a solvothermal method using Ti(SO4)2 and graphene oxide (GO) in aqueous/ethanol suspension and their microstructure and optical and photocatalytic properties investigated. The results show that, for all cases, graphene oxide was partially reduced to RGO during the solvothermal process, and anatase TiO2 nanoparticles (~ 10 nm) accumulated on the surface of RGO sheets. The formal valence state Ti4+ was identified in all cases, whereas Ti–O–C bond can form in the TiO2–RGO nanocomposites due to the strong chemical bonding effect. On increasing the mass ratio (x) of GO/Ti(SO4)2, the bandgap of the nanocomposites became narrow, thus extending the absorption wavelength range. TiO2–RGO nanocomposites exhibited good photogenerated electron transfer properties as proven by photoluminescence spectra. However, excessive addition of GO (x ≥ 1/40) decreased the photoelectron transfer performance. The photocatalytic activity was studied under ultraviolet (UV) and visible light, being greatly improved with addition of GO at x = 3/200 then decreased at x ≥ 1/40. TiO2–RGO nanocomposite (x ≤ 3/200) also exhibited better photocatalytic performance than pure TiO2 under visible light, indicating increased absorption.
Similar content being viewed by others
References
Y.M. Cui, Chin. J. Rare Met. 1, 107 (2006).
Y.M. Cui, J. Henan Univ. Sci. Technol. Nat. Sci. 1, 94 (2003).
W.R. Shen, W.K. Zhao, F.F. He, and L. You, Prog. Chem. 4, 349 (1998).
W.W. Zhang, H.L. Guo, H.Q. Sun, and R.C. Zeng, Appl. Surf. Sci. 382, 128 (2016).
O. Iieperuma, K. Tennakone, and W. Dissanayake, Appl. Catal. 62, 1 (1990).
W. Choi, A. Termin, and M.R. Hoffmann, J. Phys. Chem. 98, 13669 (1994).
R. Vogel, P. Hoyer, and H. Weller, J. Phys. Chem. 98, 3183 (1994).
H. Sedrati, R. Bensaha, H. Bensouyad, P. Miska, and S. Robert, Mater. Res. Bull. 57, 287 (2014).
L. Li and H. Xiang, Mater. Sci. Forum 610–613, 382 (2009).
D. Mo and D. Ye, Surf. Coat. Technol. 203, 1154 (2009).
M. Inagaki, F. Kojin, B. Tryb, M. Toyoda, T. Tsumura, and M. Inagaki, Carbon 43, 1652 (2005).
P.F. Du, L.X. Song, J. Xiong, N. Li, L.J. Wang, Z.Q. Xi, N.Y. Wang, L.H. Gao, and H.L. Zhu, Electrochim. Acta 87, 651 (2013).
Y.L. Pang and A. Zuhairi, Chem. Eng. J. 214, 129 (2013).
S. Morales-Torres, L.M. Pastrana-Martínez, J.L. Figueiredo, J.L. Faria, and A.M.T. Silva, Environ. Sci. Pollut. Res. 19, 3676 (2012).
W.Q. Fan, Q.H. Lai, Q.H. Zhang, and Y. Wang, J. Phys. Chem. C 115, 10694 (2011).
D.T. Wang, X. Li, J.F. Chen, and X. Tao, Chem. Eng. J. 198–199, 547 (2012).
L.M. Pastrana-Martínez, S. Morales-Torres, V. Likodimos, J.L. Figueiredo, J.L. Faria, P. Falaras, and A.M.T. Silva, Appl. Catal. B Environ. 123–124, 241 (2012).
L.J. Luo, Y. Yang, A. Zhang, M. Wang, Y.J. Liu, L.C. Bian, F.Z. Jiang, and X.J. Pan, Appl. Surf. Sci. 353, 469 (2015).
P. Calza, C. Hadjicostas, V.A. Sakkas, M. Sarro, C. Minero, C. Medana, and T.A. Albanis, Appl. Catal. B Environ. 183, 96 (2016).
P. Wang, J. Wang, X.F. Wang, H.G. Yu, J.G. Yu, M. Lei, and Y.G. Wang, Appl. Catal. B Environ. 132–133, 452 (2013).
C.P. Athanasekou, S. Morales-Torres, V. Likodimos, and G.E. Ramanos, Appl. Catal. B Environ. 158–159, 361 (2014).
G. Williams, B. Seger, and P.V. Kamat, ACS Nano 2, 1487 (2008).
I.V. Lightcap, T.H. Kosel, and P.V. Kamat, Nano Lett. 10, 577 (2010).
B. Li, X. Zhang, X. Li, L. Wang, R. Han, B. Liu, W. Zheng, X. Li, and Y. Liu, Chem. Commun. 46, 3499 (2010).
T. Xu, L. Zhang, H. Cheng, and Y. Zhu, Appl. Catal. B Environ. 101, 382 (2011).
X.Y. Zhang, H.P. Li, X.L. Cui, and Y. Lin, J. Mater. Chem. 20, 2801 (2010).
H. Zhang, X. Lv, Y. Li, Y. Wang, and J.H. Li, ACS Nano 4, 380 (2010).
Y. Zhang, Z.R. Tang, X. Fu, and Y.J. Xu, ACS Nano 4, 7303 (2010).
Y. Liang, H. Wang, H.S. Casalongue, Z. Chen, and H. Dai, Nano Res. 3, 701 (2010).
L. Liu, C. Luo, J. Xiong, Z.X. Yang, Y.B. Zhang, Y.X. Cai, and H.S. Gu, J. Alloys Compd. 690, 771 (2017).
G.P. Patil, V.S. Bagal, C.R. Mahajan, V.R. Chaudhari, S.R. Suryawanshi, M.A. More, and P.G. Chavan, Vacuum 123, 167 (2016).
P. Cheng, Z. Yang, H. Wang, W. Cheng, M. Chen, W. Shangguan, and G. Ding, Int. J. Hydrogen Energy 37, 2224 (2012).
H. Li and X.L. Cui, Int. J. Hydrogen Energy 39, 19877 (2014).
Y.Y. Gao, X.P. Pu, D.F. Zhang, G.Q. Ding, X. Shao, and J. Ma, Carbon 50, 4093 (2012).
Q.J. Xiang, J.G. Yu, and M. Jaroniec, Nanoscale 3, 3670 (2011).
Z. Zhang, W.S. Yang, X.X. Zou, F.G. Xu, X.D. Wang, B.L. Zhang, and J.L. Tang, J. Colloid Interface Sci. 386, 198 (2012).
A.A. Ismail, R.A. Geioushy, H. Bouzid, S.A. Al-Sayari, A. Al-Hajry, and D.W. Bahnemann, Appl. Catal. B Environ. 129, 62 (2013).
A.A. Galuska, J.C. Uht, P.M. Adams, and J.M. Coggi, J. Vac. Sci. Technol. A 6, 2403 (1988).
A.Y. Stakheev, E.S. Shpiro, and J. Apijok, J. Phys. Chem. 97, 5668 (1993).
V.V. Atuchin, T.A. Gavrilova, J.C. Grivel, and V.G. Kesler, J. Phys. D Appl. Phys. 42, 035305 (2009).
V.V. Atuchin, V.G. Kesler, N.V. Pervukhina, and Z. Zhang, J. Electron Spectrosc. Relat. Phenom. 152, 18 (2006).
V.V. Atuchin, L.I. Isaenko, V.G. Kesler, L. Kang, Z. Lin, M.S. Molokeev, A.P. Yelisseyev, and S.A. Zhurkov, J. Phys. Chem. C 117, 7269 (2013).
K.H. Leong, P. Monash, S. Ibrahim, and P. Saravanan, Sol. Energy 101, 321 (2014).
S. Pei and H. Cheng, Carbon 50, 3210 (2012).
S. Park, D.A. Dikin, S.T. Nguyen, and R.S. Ruoff, J. Phys. Chem. C 113, 15801 (2009).
Z. Fan, W. Kai, J. Yan, T. Wei, L. Zhi, J. Feng, Y. Ren, L. Song, and F. Wei, ACS Nano 5, 191 (2011).
C. Nethravathi and M. Rajamathi, Carbon 46, 1994 (2008).
N.R. Khalid, E. Ahmed, Z.L. Hong, and M. Ahmad, Appl. Surf. Sci. 263, 254 (2012).
P.F. Wang, Y.H. Ao, C. Wang, J. Hou, and J. Qian, J. Hazard. Mater. 223–224, 79 (2012).
D.Y. Liang, C. Cui, H.H. Hu, Y.P. Wang, S. Xu, B.L. Ying, P.G. Li, B.Q. Lu, and H.L. Shen, J. Alloys Compd. 582, 236 (2014).
J. Hu, H.S. Li, S. Muhammad, Q. Wu, Y. Zhao, and Q.Z. Qiao, J. Solid State Chem. 253, 113 (2017).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51602279), Jiangsu Planned Projects for Postdoctoral Research Funds (1601048B), Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middleaged Teachers and Presidents, and the Program for High-end Talents in Yangzhou University, Outstanding Young Backbone Teacher Project of Yangzhou University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D., Dai, S., Li, J. et al. Microstructure and Photocatalytic Properties of TiO2–Reduced Graphene Oxide Nanocomposites Prepared by Solvothermal Method. J. Electron. Mater. 47, 7372–7379 (2018). https://doi.org/10.1007/s11664-018-6677-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6677-8