Abstract
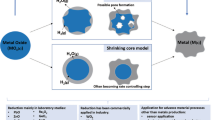
Rotary kiln-electric furnace (RKEF) technology accounts for 95 pct of the lateritic nickel ore processing. Coal is commonly used as fuel and reducing agent in the rotary kiln to produce solid reduction product which is then melted in the electric furnace. The use of coal in the process leads to the emission of approximately 70 tons of CO2 for every 1 ton of nickel in ferronickel or nickel pig iron product. One of the possible solutions for minimizing CO2 emission in the RKEF process is through the substitution of carbon-based fuel and reducing agent with hydrogen gas. This research focuses on experimental investigation on the reduction of saprolite nickel ore under hydrogen-argon atmosphere. The research was initiated by performing a thermodynamic simulation using FactSage 8.0 to preliminary evaluate the effects of different parameters on the reduction process. Reduction experiments of saprolite nickel ore were then carried out in a horizontal tube furnace with varying temperature (500 °C, 600 °C, 700 °C, 800 °C, and 900 °C), hydrogen gas composition (25 and 75 pct H2 in H2–Ar gas mixture), and reaction time (0.25 to 3 hours) to simulate the process in the rotary kiln. The solid reduction products were analyzed using ICP-MS, SEM-EDS, and XRD. The overall reduction extents in all conditions were also monitored by weight measurements of the samples before and after the reduction processes. Based on the weight changes, the reduction process was found to be significantly rapid so that it reached completion in 15 minutes. Each solid reduction product was then melted in a vertical tube furnace at 1550 °C for 2 hours under inert, argon atmosphere to simulate the process in the electric furnace. The metal product after melting had an average composition of 80.6 pct Fe and 8.3 pct Ni which is similar to commercially produced nickel pig iron.











Similar content being viewed by others
References
U.S. Geological Survey: Mineral commodity summaries 2023 (2023), https://www.usgs.gov/. Accessed 3 June 2023
D.A. Quintero-Coronel, W.D. Guillin-Estrada, J.L. Echeverri-Roman, H. Maury, L. Corredor, J.A. Ruiz, B.S. Rueda, and A. Gonzalez-Quiroga: Therm. Sci. Eng. Prog., 2022, vol. 32, pp. 1–14.
F. Crundwell, M. Moats, V. Ramachandran, T. Robinson, and W.G. Davenport: Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier, Oxford, 2011.
M. Mistry, J. Gediga, and S. Boonzaier: Int. J. Life Cycle Assess., 2016, vol. 21, pp. 1559–72.
Q. Jeangros, T.W. Hansen, J.B. Wagner, C.D. Damsgaard, R.E. Dunin-Borkowski, C. Hébert, J. Van Herle, and A. Hessler-Wyser: J. Mater. Sci., 2013, vol. 48, pp. 2893–2907.
T. Hidayat, M.A. Rhamdhani, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 474–89.
B. Janković, B. Adnađević, and S. Mentus: Chem. Eng. Sci., 2008, vol. 63, pp. 567–75.
T. Hidayat, M.A. Rhamdhani, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 1–16.
J. Szekely and J.W. Evans: Chem. Eng. Sci., 1971, vol. 26, pp. 1901–13.
T. Hidayat, M.A. Rhamdhani, E. Jak, and P.C. Hayes: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 462–73.
V. de Alvarenga Oliveira, R. de Jesus Taveira Lana, H.C. da Silva Coelho, G.J. Silva Brigolini, and C.G. Dos Santos: Metall. Mater. Trans. B, 2020B, vol. 51B, pp. 1418–31.
J.J. Wijenayake, S.Y. Lee, S.H. Park, and H.S. Sohn: Hydrometallurgy, 2021, vol. 203, p. 105622.
S. Liu, C. Yang, S. Yang, Z. Yu, Z. Wang, K. Yan, J. Li, and X. Liu: Front. Chem., 2021, vol. 9, p. 704012.
J. Chen, E. Jak, and P.C. Hayes: Miner. Process. Extr. Metall., 2021, vol. 130, pp. 148–59.
J. Chen, E. Jak, and P.C. Hayes: Miner. Process. Extr. Metall., 2021, vol. 130, pp. 160–69.
J. Chen, E. Jak, and P.C. Hayes: Miner. Process. Extr. Metall., 2021, vol. 130, pp. 170–79.
J. Chen, E. Jak, and P.C. Hayes: Miner. Process. Extr. Metall., 2021, vol. 130, pp. 425–32.
W. Mayangsari, E. Febriana, and A.B. Prasetyo: AIP Conference Proceedings, No. 1964, pp. 020014-1–0200146, AIP Publishing, 2018.
D. Zhu, L. Pan, Z. Guo, J. Pan, and F. Zhang: Adv. Powder Technol., 2019, vol. 30, pp. 451–60.
M.H. Morcali, L.T. Khajavi, and D.B. Dreisinger: Int. J. Miner. Process., 2017, vol. 167, pp. 27–34.
Y. Zhang, J. Qie, X.F. Wang, K. Cui, T. Fu, J. Wang, and Y. Qi: Mining Metall. Explor., 2020, vol. 37, pp. 79–91.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R.B. Mahfoud, J. Melançon, A.D. Pelton, and S. Petersen: Calphad, 2002, vol. 26, pp. 189–228.
G.W. Brindley and R. Hayami: Mineral. Mag. J. Mineral. Soc., 1965, vol. 35, pp. 189–95.
Acknowledgments
This work was financially supported by the Bandung Institute of Technology (ITB) Flagship Research Program 2022. The authors would like to acknowledge the support from PT Gunbuster Nickel Industry for providing the Scanning Electron Microscope-Energy Dispersive X-ray Spectroscopy (JCM-7000 NeoScopeTM, JEOL, Tokyo, Japan) used for the analysis of the samples in this work. The authors would also like to acknowledge the support from the Department of Mineral Sciences of the Smithsonian Institution for providing Basaltic Glass and Springwater Olivine reference materials and Research & Development Center tekMIRA of Indonesia for providing the ore preparation facility.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satritama, B., Zulhan, Z., Wulandari, W. et al. The Impacts of Temperature, Gas Composition and Reaction Time on the Reduction of Saprolite Nickel Ore Under Hydrogen–Argon Atmosphere. Metall Mater Trans B 55, 396–408 (2024). https://doi.org/10.1007/s11663-023-02965-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-023-02965-4