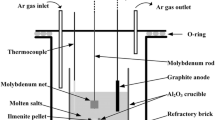
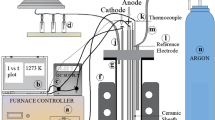
Abstract
The electrochemical behaviors of boron species on Mo and Ni electrodes in molten NaCl–CaCl2–CaO–B2O3 at 1123 K were investigated to establish a process for CaB6 production using molten salt electrolysis. Electrolysis was performed at four different potentials (− 2.97, − 2.77, − 2.37, and − 2.07 V vs Cl−/Cl2) on Mo and Ni plates. Boronized Mo or Ni was observed under all experimental conditions. At a potential of − 2.37 V, the boronized layer was a few micrometers thick for Mo, whereas it was approximately 15 µm for Ni under the same electrolytic conditions. Moreover, CaB6 was formed at − 2.37 V or a more negative potential in the case of Mo, although it was formed at − 2.77 V or a more negative potential in the case of Ni. Boronizing proceeded more rapidly on Ni than on Mo, indicating that boronizing tends to be predominant over the formation of CaB6 in the case of Ni. The results show that Mo is a more suitable electrode material than Ni for CaB6 production. Furthermore, even though the melt contains Ca2+ ions, the metal can be boronized under appropriate conditions by molten salt electrolysis without the formation of CaB6.











Similar content being viewed by others
References
Z. Yahia, S. Turrell, G. Turrell, and J.P. Mercurio: J. Mol. Struct., 1990, vol. 224, pp. 303–12.
H. Yin, D. Tang, X. Mao, W. Xiao, and D. Wang: J. Mater. Chem. A, 2015, vol. 3, p. 15184.
K. Giannò, A.V. Sologubenko, H.R. Ott, A.D. Bianchi, and Z. Fisk: J. Phys. Condens. Matter, 2002, vol. 14, pp. 1035–43.
S. Otani: J. Cryst. Growth, 1998, vol. 192, pp. 346–49.
M. Takeda, M. Terui, N. Takahashi, and N. Ueda: J. Solid State Chem., 2006, vol. 179, pp. 2823–26.
J.L. Andrieux: Ann. Chim., 1929, vol. 10, p. 423.
K. Uchida: Surf. Technol., 1978, vol. 7(1), pp. 39–44.
X. Wang and Y. Zhai: J. Appl. Electrochem., 2009, vol. 39, pp. 1797–1802.
Y. Chernov, E. Filatov, N. Shurov, V. Smolenski, and N. Tkachev: Metall. Mater. Trans. B, 2019, vol. 50, pp. 1745–51.
S. Angappan, M. Helan, A. Visuvasam, L.J. Berchmans, and V. Ananth: Ionics, 2011, vol. 17, pp. 527–33.
D. Chukhvantsev, E. Filatov, and N. Shurov: Mater. Sci. Eng. B, 2022, vol. 284, p. 115917.
W. Weng, M. Wang, X. Gong, Z. Wang, D. Wang, and Z. Guo: J. Electrochem. Soc., 2018, vol. 165, p. E477.
Y. Katasho, K. Yasuda, and T. Nohira: J. Nucl. Mater., 2018, vol. 503, pp. 290–303.
X. Chen, Y. Zhang, J. Qu, X. Qu, B. Zhang, Z. Zhao, Y. Zhao, D. Wang, and H. Yin: Sep. Purif. Technol., 2022, vol. 285, p. 120391.
M. Kulka: Current Trends in Boriding, Springer, Cham, 2019, pp. 48–58.
L. Segers, A. Fontana, and R. Winand: Electrochim. Acta., 1991, vol. 36(1), pp. 41–47.
G. Kartal, S. Timur, and C. Arslan: J. Electron. Mater., 2005, vol. 34(12), pp. 1538–42.
V. Sista, O. Kahvecioglu, G. Kartal, Q.Z. Zeng, J.H. Kim, O.L. Eryilmaz, and A. Erdemir: Surf. Coat. Technol., 2013, vol. 215, pp. 452–59.
O.K. Feridun, V. Sista, O.L. Eryilmaz, and A. Erdemir: Surf. Eng., 2015, vol. 31, pp. 575–80.
H. Okamoto: J. Phase Equilib. Diffus., 2018, vol. 39(6), pp. 953–65.
M. Tada and Y. Ito: Denki Kagaku oyobi Kogyo Butsuri Kagaku, 1992, vol. 60, pp. 515–22.
Y. Gu, et al.: J. Alloys Compd., 2017, vol. 690, pp. 228–38.
X. Yang, L. Ji, X. Zou, T. Lim, J. Zhao, E.T. Yu, and A.J. Bard: Angew. Chem., 2017, vol. 56, pp. 15078–82.
G.K. Sireli: Encyclopedia of Iron, Steel, and Their Alloys, Taylor & Francis, New York, 2015, pp. 2284–2300.
K. Koyama, H. Shimotake, and F.C. Mrazek: J. Electrochem. Soc., 1983, vol. 130, pp. 147–51.
D. Killinger and S. Phongikaroon: J. Electrochem. Soc., 2021, vol. 168, p. 036518.
The Chemical Society of Japan, Kagaku binran kisohen, 6th edition, Maruzen, 2020.
Y. Katasho, K. Yasuda, and T. Nohira: J. Electrochem. Soc., 2017, vol. 164, pp. D478–85.
Y. Seto and M. Ohtsuka: J. Appl. Cryst., 2022, vol. 55, pp. 397–410.
Material Property Data, https://www.matweb.com/search/datasheet.aspx?matguid=f2e8eea2aeee449e9ab92f1a0302da12&ckck=1. Accessed 6 March 2023.
A. Jain, S.P. Ong, G. Hautier, W. Chen, W.D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder, and K.A. Persson: APL Mater., 2013, vol. 1, p. 011002.
H. Li, Y. Fu, J. Liang, and Y. Yang: Materials, 2022, vol. 15(21), p. 7646.
Y. Zaikov, V. Batukhtin, N. Shurov, and A. Suzdaltsev: Electrochem Mater. Technol., 2022, vol. 1, pp. 1–21.
A.V. Suzdaltsev, et al.: J. Electrochem. Soc., 2017, vol. 164, pp. H5183–88.
Y.P. Zaikov, V.P. Batukhtin, N.I. Shurov, L.E. Ivanovskii, and A.V. Suzdaltsev: Metall. Mater. Trans. B, 2014, vol. 45, pp. 961–67.
Y.P. Zaikov, N.I. Shurov, V.P. Batukhtin, and O.G. Molostov: Metall. Mater. Trans. B, 2014, vol. 45, pp. 968–74.
A. Mukherjee, R. Kumaresan, and S. Ghosh: J. Electroanal. Chem., 2021, vol. 902, p. 115778.
Japan new metals Co. Ltd, The physical properties of a compound: Specific Electrical Conductivity <http://www.jnm.co.jp/en/data/electrical_conductivity.html> Accessed 19 Sep 2023.
Acknowledgments
This study was partially supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan and JSPS KAKENHI (Grant Number 19K23582).
Conflict of interest
A conflict of interest exists whenever an author has a financial or personal relationship with a third party whose interests could be positively or negatively influenced by the article's content. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katasho, Y., Oishi, T. & Haarberg, G.M. Electrochemical Formation of Calcium Hexaboride and Boronizing of Metal Electrodes in CaCl2-Based Molten Salt. Metall Mater Trans B 55, 266–277 (2024). https://doi.org/10.1007/s11663-023-02956-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-023-02956-5